Protozoan Infections of the Thorax
AMEBIASIS: ENTAMOEBA HISTOLYTICA
Entamoeba histolytica is a protozoan infection of humans that is found worldwide and is globally responsible for up to 100,000 deaths annually. It is endemic in most temperate and tropical areas of the world, particularly in areas with poor socioeconomic development and limited sanitation. Serologic evidence of prior or current infection with E. histolytica is present in 5% to 50% of individuals in impoverished populations. E. histolytica is infectious in the cyst form. Transmission usually occurs as a result of contamination of food or water, but may also occur by means of oral–anal contact. Infection is common in developing countries. In Europe and the United States, infection is most commonly seen in individuals who have lived in endemic areas of the world. Institutionalized individuals are also at increased risk of infection. Sexually active men who have sex with men are also at increased risk of infection. There does not appear to be an increased risk of invasive disease in persons with HIV infection.
A morphologically identical, but nonpathogenic protozoan, Entamoeba dispar, also infects the human gastrointestinal tract. It can be distinguished from E. histolytica by antigenic, genetic, and immunologic methods. Infection is 10 times more prevalent with E. dispar than with E. histolytica; however, the former does not cause invasive disease. Many older epidemiologic studies relied on microscopic diagnosis alone, and inadvertently incorporated both pathogenic and nonpathogenic species in estimates of prevalence of infection.
E. histolytica has a simple life cycle involving an infectious cyst and an ameboid trophozoite phase. Cysts may survive in the external environment for several weeks to months, especially in damp conditions and temperatures between –5°C and 40°C. After ingestion by humans, cysts excyst in the small intestine, each forming eight daughter trophozoites. These motile trophozoites can adhere to the intestinal wall; it is in this form that they may invade the mucosa, causing symptomatic invasive disease. In the colon, trophozoites encyst to complete the life cycle and are excreted. Trophozoites do not survive outside the human host.
 CLINICAL MANIFESTATIONS
CLINICAL MANIFESTATIONS
The incubation period for intestinal amebiasis is usually 1 to 4 weeks, but ranges from a few days to months. Infection with E. histolytica is asymptomatic in up to 90% of cases, but may cause a range of gastrointestinal symptoms from mild diarrhea to severe colitis with bloody diarrhea. Symptoms result from penetration of the trophozoites through the mucosal barrier with invasion of the colon wall. In the absence of frank blood, stool is usually positive for occult blood. Fever is present in less than half of cases. Fecal leukocytosis is usually less marked than in bacterial colitis. Rarely, progression of invasive intestinal disease may lead to severe colitis and bowel perforation. On occasion, granulation tissue formation surrounding a localized area of invasive disease may form an inflammatory mass (an “ameboma”) and manifest as a tender abdominal mass.
Extraintestinal disease most commonly occurs in the liver, and manifests as a liver “abscess.” In this instance, trophozoites migrate to the liver via the portal veins, and infection results in inflammation, necrosis, and ultimately a fluid collection. The collection is often referred to as an abscess; however, it does not contain polymorphonuclear cells like a true abscess, but only cellular debris. Eighty percent of amebic hepatic abscesses are solitary, and 80% occur in the right lobe of the liver. Extraintestinal disease may present years after residence in an endemic area and should be considered in all patients with an appropriate travel history and suggestive symptoms. Liver abscesses occur more frequently in men than women, and usually in the age range of 18 to 50 years.
Thoracic manifestations of E. histolytica infection are rare; they have been reported in 2% to 3% of cases of invasive amebiasis. They can be considered chiefly as either pleuropulmonary or pericardial. A case of E. histolytica osteomyelitis of the rib has been reported, as has a case of pulmonary amebiasis presenting as superior vena cava syndrome.1
 PLEUROPULMONARY DISEASE
PLEUROPULMONARY DISEASE
Amebic pulmonary disease may occur by a number of mechanisms.1–3 It most often occurs as the result of a concomitant liver abscess. The abscess may result in a sympathetic pleural effusion that does not require specific therapy. A liver abscess may alternatively rupture through the diaphragm into the pleural space, causing respiratory distress, empyema, and subsequent parenchymal lung abscesses (Figs. 136-1 to 136-3). Pulmonary parenchymal disease in the form of lung abscesses and consolidation less often occurs in the absence of hepatic disease, but may occur if there has been hematogenous spread of trophozoites to the lung.
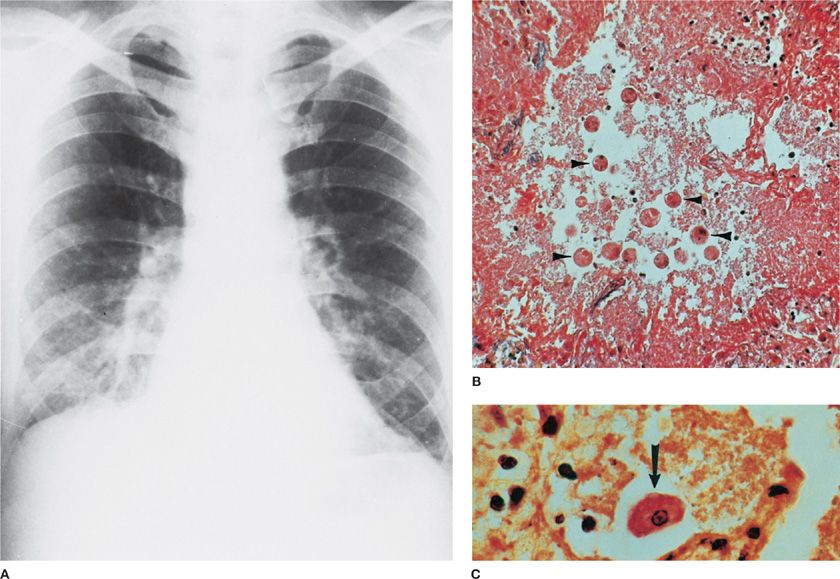
Figure 136-1 Entamoeba histolytica involving the right lung after rupture of a hepatic abscess through the right hemidiaphragm. A. Chest radiograph shows elevated right hemidiaphragm, right lower lobe infiltrate, and effusion. (Used with permission of Armed Forces Institute of Pathology [AFIP].) B. Alveolar spaces are filled with ameba (arrowheads). (Iron hematoxylin stain, ×300). (Used with permission of Dr. Y. Gutierrez.) C. Trophozoite (arrow) and necrotic debris.
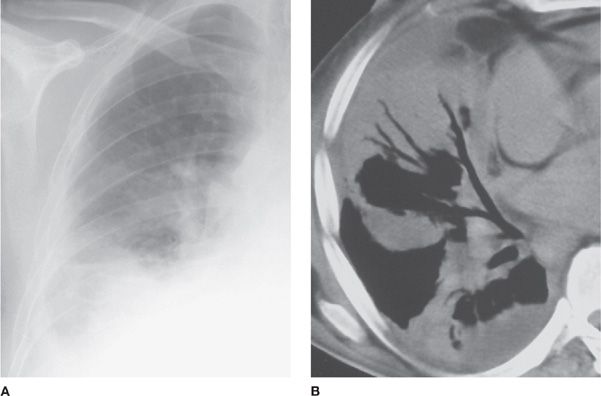
Figure 136-2 Right-sided amebic pleural empyema and pneumonia in a 43-year-old man with an abscess of the right hepatic lobe. A. Initial chest radiograph shows pleural effusion and right-sided basal consolidation. B. Chest CT scan helps confirm pleural involvement and right-sided basal alveolar infiltrates. Trophozoites of E. histolytica were obtained at bronchoalveolar lavage. (Reproduced with permission from Martinez S, Restrepo CS, Carrillo JA, et al. Thoracic manifestations of tropical infections: a pictorial review. RadioGraphics. 2005;25(1):135–155.)
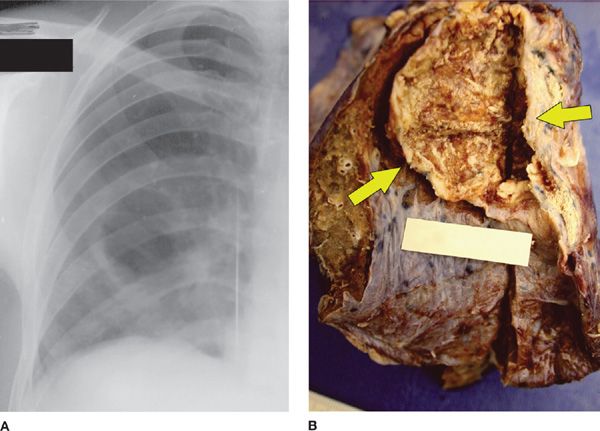
Figure 136-3 Right-sided amebic pneumonia in a 35-year-old man with a hepatic abscess. A. Chest radiograph shows elevation of the right hemidiaphragm and right-sided basal consolidation with cavitation. B. Photograph of the gross specimen demonstrates an irregular cavitary lesion (arrows). Anchovy sauce content and trophozoites of E. histolytica (not shown) were found on the lesion wall. (Reproduced with permission from Martinez S, Restrepo CS, Carrillo JA, et al. Thoracic manifestations of tropical infections: a pictorial review. RadioGraphics. 2005;25(1):135–155.)
Most patients with extraintestinal amebiasis have not had symptomatic intestinal disease, so the absence of preceding gastrointestinal symptoms does not exclude the diagnosis. Patients with liver abscess often present with insidious onset of fever, right upper quadrant pain, and hepatic tenderness on palpation. Pleuritis, respiratory symptoms such as cough, dyspnea, and/or physical examination consistent with pleural effusion further suggest involvement of the pleuropulmonary system. Sputum production may range from scant to copious, and sputum may contain purulent material, particularly if a hepatobronchial fistula has developed. Classically, the purulent material of amebic abscess is reddish “anchovy paste.” Jaundice is uncommon. Leukocytosis may be present, but eosinophilia is not a feature of the disease.
 PERICARDIAL DISEASE
PERICARDIAL DISEASE
Pericardial disease can result from either sterile inflammation of the pericardium as a result of a contiguous liver abscess in the left lobe of the liver, or may be a purulent infection as a result of rupture of a hepatic amebic abscess into the pericardial space (Fig. 136-4). Chest pain, pericardial friction rub, and symptoms and signs of pericarditis may be presenting features. Presentation may be acute and severe, with symptoms and signs of pericardial tamponade.4
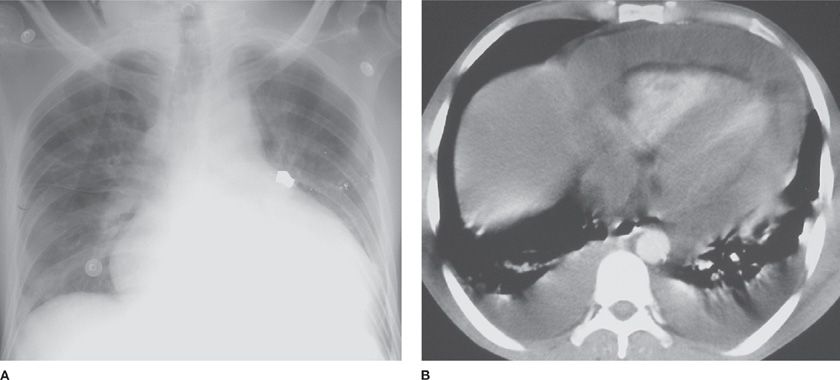
Figure 136-4 Amebic pericardial tamponade in a 27-year-old man with an abscess of the left hepatic lobe. The patient presented with pulsus paradoxus, fever, and chills. A. Chest radiograph shows enlargement of the cardiac silhouette. B. Chest CT scan shows extensive pericardial effusion that resulted from rupture of a left hepatic lobe abscess (not shown) into the pericardial space. (Reproduced with permission from Martinez S, Restrepo CS, Carrillo JA, et al. Thoracic manifestations of tropical infections: a pictorial review. RadioGraphics. 2005;25(1):135–155.)
 DIAGNOSIS OF PLEUROPULMONARY AND PERICARDIAL DISEASE
DIAGNOSIS OF PLEUROPULMONARY AND PERICARDIAL DISEASE
Stool microscopy may reveal the presence of E. histolytica trophozoites or cysts, but microscopy is neither a sensitive nor a specific method for the diagnosis of amebiasis. Microscopy is unable to distinguish E. histolytica from nonpathogenic E. dispar, and extraintestinal disease is associated with presence of the pathogen in stool in only a minority of cases. Stool may be concentrated and stained with iodine to evaluate for the presence of cysts. Trophozoites are best seen on a fresh smear with iron hematoxylin and trichome stain. Microscopy of abscess fluid occasionally may reveal presence of trophozoites. Diagnosis of extraintestinal disease is best made by a combination of microscopic examination of samples, antigen detection, and serology tests.
 ANTIGEN DETECTION
ANTIGEN DETECTION
Antigen detection methods detect E. histolytica. These tests are able to distinguish E. dispar from E. histolytica. Stool antigen tests are over 95% sensitive and specific for the diagnosis of intestinal disease. Antigen detection assays are also highly sensitive and specific when applied to abscess fluid.
 SEROLOGY
SEROLOGY
Most patients infected with E. histolytica, but not E. dispar, develop a serum antibody response. Amebic serology is positive in over 90% of patients with extraintestinal disease. Serologic analysis may be negative in early infection. The presence of IgG antibodies does not always indicate active disease; IgG may persist for many years after active infection.
 TREATMENT
TREATMENT
Metronidazole or tinidazole is effective in the treatment of individuals with intestinal and extraintestinal amebiasis. To prevent a relapse, a luminal agent such as iodoquinol or paromomycin also should be administered. Sympathetic pleural effusions do not need specific therapy, other than treatment of the underlying liver abscess. Amebic empyema and amebic pericarditis are best treated with aspiration or percutaneous drainage, in combination with administration of antiamebic microbial therapy.
FREE-LIVING AMEBA: ACANTHAMOEBA
Free-living amebae are rarely associated with human infection; however, when infection does occur, disease tends to be progressive and difficult to treat.
Acanthamoeba spp. are ubiquitous, and have been isolated from a wide variety of locations, including the nasal passages of healthy adults, water sources, soil, and contact lens fluid. Infection in humans usually manifests as either keratitis or systemic disease. Systemic disease usually involves the central nervous system, manifesting as granulomatous amebic encephalitis. Amebic infection of skin also has been reported.
 CLINICAL FEATURES
CLINICAL FEATURES
Acanthamoeba sp. keratitis is usually a disease of healthy individuals, often contact lens wearers. Infection is thought to result from direct deposition of ameba into the eye. In contrast, systemic disease results from hematogenous dissemination of ameba, which are thought to first enter the human host via the respiratory tract or skin. Systemic disease is primarily a disease of immunocompromised or debilitated persons, including individuals with acquired immunodeficiency syndrome (AIDS), and those who have undergone bone marrow transplantation, organ transplantation, or are otherwise immunosuppressed. Pulmonary nodular infiltrates and pneumonitis have been reported in cases of systemic disease.5
 DIAGNOSIS
DIAGNOSIS
Diagnosis of systemic acanthamebiasis is usually made late in the clinical course, and unfortunately, very often at autopsy. Evaluation of cerebrospinal fluid from individuals with granulomatous amebic meningoencephalitis may disclose hypoglycorrhachia and elevated protein, but amebae are not usually seen. Evaluation of biopsies of lesions of skin or brain usually provides the definitive diagnosis. Acanthamoeba spp. can be identified on histopathology, and may be cultured on nutrient agar overlaid with Escherichia coli. Radiographic imaging of the central nervous system is often helpful.
 TREATMENT
TREATMENT
No single drug is effective against systemic Acanthamoeba spp. infection. Combinations of treatment with amphotericin B, azithromycin, voriconazole, fluconazole, 5-fluorocytosine, pentamidine, and sulfadiazine have been attempted, with success in some cases and failure in other cases that used similar regimens. Most individuals with systemic amebiasis do not survive, regardless of therapy. Individuals with acanthamebic keratitis are usually treated topically and may require corneal grafting.
SYSTEMIC COCCIDIOSIS: TOXOPLASMOSIS
Toxoplasmosis is caused by Toxoplasma gondii. Asymptomatic disease is common. Symptomatic disease usually occurs in persons with suppressed cell-mediated immunity and infants born with congenital infection.
 EPIDEMIOLOGY AND LIFE CYCLE
EPIDEMIOLOGY AND LIFE CYCLE
Understanding the life cycle of T. gondii is important in understanding the disease process. The parasite is found worldwide. It has both sexual and asexual stages, the latter being the pathogenic stage in humans and other animals. Cats are the definitive host, and in the feline, oocysts develop after an intestinal intraepithelial sexual cycle. Oocysts are then passed in cat feces, and after 2 to 3 days become infectious. They may persist in soil. If ingested by humans or other animals (sheep, pigs, rodents, or cats), oocysts produce rapidly dividing tachyzoites. Tachyzoites invade varying cell types, and result in cell death and tissue necrosis. Eventually the host immune response curtails this process, and tachyzoites give rise to slowly dividing bradyzoites, which encyst and persist indefinitely in host tissues. Bradyzoites are infectious if ingested, giving rise to tachyzoites in the new host. Bradyzoites may also revert to tachyzoites if a host becomes immunocompromised, leading to “reactivation” of disease. Humans and other animals may be infected, not only by ingestion of oocysts, but also by ingestion of tissue cysts, such as following the consumption of undercooked infected meat. Toxoplasma infection may also be acquired by transplacental passage of tachyzoites, resulting in congenital infection, or by organ transplantation.
Serologic studies demonstrate a wide geographic variation in prevalence of infection between 10% and 90%. In the United States, rates range from 3% in Denver, Colorado, to 17% in Massachusetts, and 30% in Birmingham, Alabama.
 CLINICAL FEATURES
CLINICAL FEATURES
Infection may be primary or due to reactivation. After ingestion of infectious oocysts or tissue cysts, tachyzoites disseminate and invade organs, replicating intracellularly. Acute infection may result in tachyzoites being found in all organs, especially muscle, lymphatic tissue, and the central nervous system. Primary infection may be subclinical or asymptomatic. If symptomatic, the most common manifestations of acute disease include fever, painless lymphadenopathy, and a mononucleosis-like syndrome. More rarely pneumonitis, myocarditis, and myositis may occur.
Congenital infection is usually the result of primary infection in the mother, with transplacental passage of tachyzoites resulting in infection of the fetus. Infected infants may be symptomatic at birth, or may have hepatosplenomegaly, pneumonitis, myocarditis, rash, or jaundice. Congenitally infected children may be asymptomatic initially, but may subsequently develop central nervous system or ocular manifestations.
Reactivation disease occurs in persons who are immunocompromised, particularly those with deficiency in cell-mediated immunity. Patients with HIV infection and CD4 cell counts less than 100 cells/mm3 are at particular risk of life-threatening disease, and although primary infection may occur in these individuals, reactivation disease is most common. Reactivation disease may be prevented in those at particular risk with the prophylactic use of trimethoprim–sulfamethoxazole. Necrotizing central nervous system disease is the most common manifestation of reactivation toxoplasmosis in AIDS patients. Affected individuals usually present with altered mental status, fever, headache, and clinical findings suggestive of focal CNS disease, and neuroimaging often reveals multifocal, ring-enhancing lesions.
 PULMONARY DISEASE
PULMONARY DISEASE
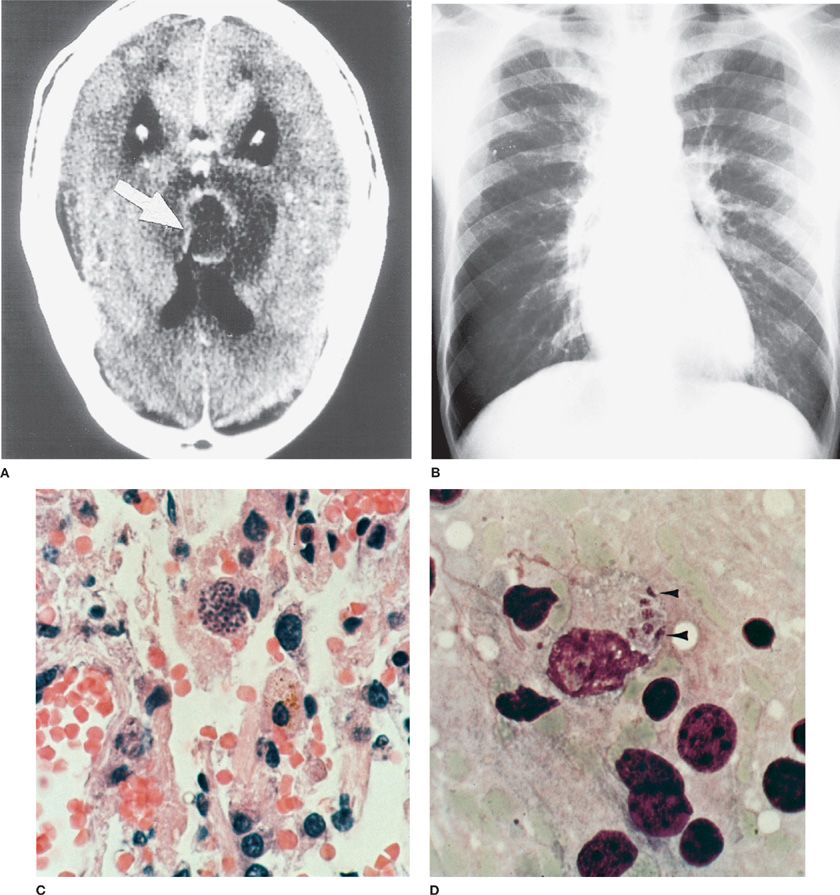
Pulmonary involvement in toxoplasmosis is common, and is usually reported in patients with AIDS or in individuals who have undergone bone marrow or solid-organ transplantation (Fig. 136-5).6,7
Figure 136-5 Toxoplasma gondii in the brain and lung of a patient with AIDS. A. Brain CT scan shows Toxoplasma abscess as a contrast-enhancing lesion (arrow). B. Chest radiography. Diffuse bilateral infiltrates and hilar adenopathy. C. Lung biopsy shows Toxoplasma forms (arrow, cyst, hematoxylin & eosin stain, ×100). D. Impression smear of brain biopsy shows five intracellular tachyzoite (trophozoite) forms (arrowheads; Giemsa stain ×1500). (C and D are Used with permission of Dr. Y. Gutierrez.)
Shortness of breath and cough are the most common presenting symptoms of pulmonary involvement during toxoplasmosis. In one review, immunocompetent patients with toxoplasmic pneumonitis were more likely than immunocompromised individuals to have evidence of hepatosplenomegaly at presentation, but clinical findings were otherwise similar. Bilateral interstitial pulmonary infiltrates are most often evident in radiography; however, a variety of other findings, from discrete pulmonary opacities to cavitary disease, also have been described. Severe disease or the acute respiratory distress syndrome (ARDS) may develop. T. gondii organisms can invade myocytes, and during acute or reactivation disease, myocarditis may occur and may be severe.
 DIAGNOSIS
DIAGNOSIS
In primary toxoplasmosis, the differential diagnosis is broad, but IgM-specific antibodies will usually be present. During reactivation of toxoplasmosis, IgM and IgG antitoxoplasma antibodies are usually present in serum. Absence of antitoxoplasma IgG antibodies makes the diagnosis of reactivation toxoplasmosis unlikely, but the presence of the antibody itself does not differentiate between latent and active diseases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree