Fig. 31.1
Bulging upper extremity collateral veins as a result of central stenosis
Additional Examination
Further upper extremity examination includes a neurologic examination in order to document the presence of neuropathy or motor and sensory dysfunction. A skin examination should document scars from prior access procedures, other operations, and trauma. The chest should also be examined for presence of scars from possible prior central venous access sites. Indications of congestive heart failure, such as jugular venous distention, result in a cardiology consultation preoperatively to maximize cardiac function prior to surgery [14].
Diagnostic Imaging
Noninvasive Arterial Imaging
If any abnormality is detected during the physical examination of the arterial inflow, subsequent evaluation starts with noninvasive imaging, beginning with segmental arterial pressures and either pulse volume recording (PVR) or duplex ultrasound. Arteriography is the gold standard for diagnosis of arterial stenosis; however, there are several limitations to consider, especially in patients with CKD. For all patients, arteriography is invasive, with risk of puncture site complications, including hematoma, pseudoaneurysm, dissection, or thrombosis. CKD patients have an added risk of contrast-induced nephropathy (CIN), which may result in the patient requiring hemodialysis at a sooner date. Preexisting renal dysfunction is the most important risk factor for the development of CIN, and the incidence increases with the degree of preprocedure renal dysfunction [15]. Therefore, arteriogram should be reserved for patients with abnormal noninvasive imaging as discussed below.
Segmental Arterial Pressure
Segmental pressures are obtained initially after an abnormal upper extremity arterial examination and are performed in a similar manner to segmental pressures in the legs. Blood pressure cuffs are placed on the upper arm, forearm, and finger to give an estimate of the level of disease in the upper extremity, and the brachial, radial, and ulnar arteries are assessed with Doppler ultrasound, while digital arteries are assessed using photo-plethysmography (PPG).
Pressures should be equal, without pressure gradient, in both extremities, as in Fig. 31.2. If the arm pressures at the same cuff level on opposite arms differ by more than 20 mmHg or the pressure measurements between adjacent cuff sites on the same arm differ by more than 10 mmHg [16], this is indicative of occlusive disease with a significant stenosis, as indicated in the right arm of Fig. 31.3. The main drawback of segmental pressures is the abnormally high values seen due to calcified vessels, which is commonly seen in diabetic patients. In this group of patients, a finger brachial index may be obtained. A normal finger/brachial index should be greater than 0.7; when the index is below 0.7, it suggests obstruction to flow and should prompt further evaluation of the upper extremity arterial system with PVRs and/or DU [17].
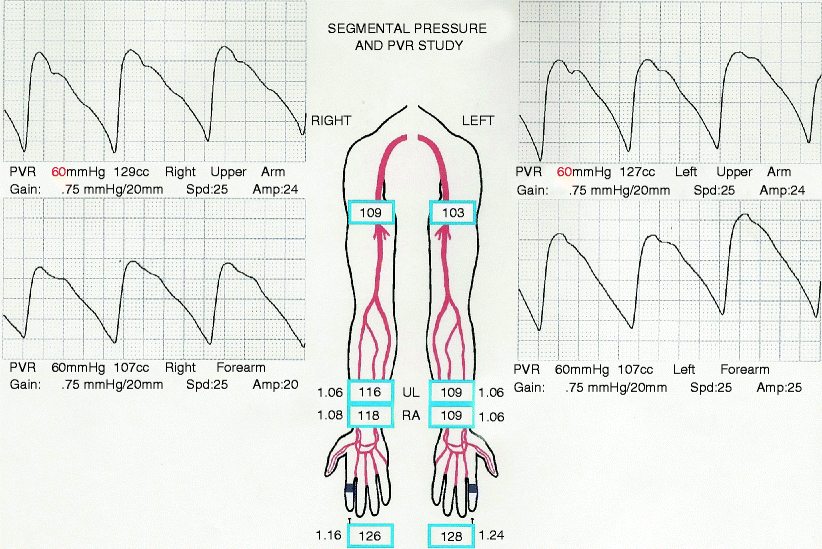
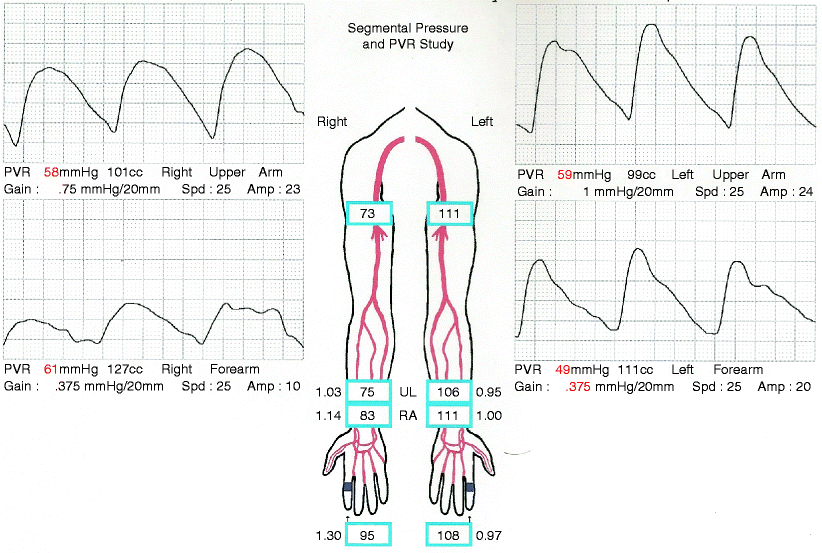

Fig. 31.2
Normal upper extremity segmental pressures and PVR. Pressures are equal bilaterally and the triphasic waveform has a strong upslope, a dicrotic notch, and a steep downslope

Fig. 31.3
An abnormal upper extremity segmental pressure and PVR. The pressure in the right upper arm is significantly lower than that on the left. The PVR on the right also has lost the dicrotic notch and shows evidence of dampening
Pulse-Volume Recording
Like segmental pressure measurements, PVR, using volume plethysmography, also uses interval blood pressure cuffs that detect the arterial pulsations and gives the sum of the blood flow in the arteries of that segment of the upper extremity [18]. This is displayed as a triphasic arterial waveform, as seen in Fig. 31.2. The normal waveform has a sharp upslope, a dicrotic notch, and a downslope. There should be no evidence of dampening, which indicates a stenosis proximal to the respective cuff [19]. When a significant stenosis is detected, the dicrotic notch is typically lost first, followed by a more shallow upstroke and, finally, an overall decreased amplitude of the arterial waveform (see Fig. 31.3). This test does not require compression of the vessels, and, therefore, these waveforms should be unaffected by arterial calcification. Any abnormality seen on the PVRs should be further evaluated by arteriography.
Duplex Ultrasonography
Color flow DU is used to assess the arterial inflow when abnormalities are detected during segmental pressures or PVRs. Using DU, the entire arterial anatomy of the upper extremity, including the subclavian artery, may be visualized and allows for evaluation of arterial diameter as well as presence of arterial stenosis or calcification, and abnormal arterial wall thickening [19] (Fig. 31.4).
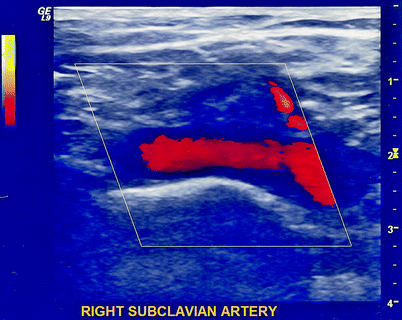

Fig. 31.4
Duplex ultrasound of a normal right subclavian artery
Multiple studies have evaluated minimum diameter necessary for successful AV access, and both 1.5 and 2.0 mm have been used as the minimally accepted internal arterial diameters; however, unobstructed inflow artery diameter of 2.0 mm has more often been used as a predictor of success [20, 21]. Lemson et al. reported that patients with failed forearm fistulas had significantly smaller mean preoperative radial artery diameters compared to patients with matured fistulas (1.9 vs. 2.8 mm) [22].
An additional benefit to DU diagnostic capabilities is its possible use for therapeutic procedures such as DU-guided angioplasty. This has been described for treatment of lower extremity occlusive disease, carotid disease, and non-maturing AV access as an alternative to standard angiography with angioplasty in an effort to avoid radiation exposure and contrast material, especially in patients with renal insufficiency [23].
Noninvasive Venous Imaging
Superificial Venous System
DU is critical to the complete evaluation of the venous outflow. All patients who have an abnormal venous examination or do not have adequately visualized superficial veins on physical examination should undergo superficial vein mapping with DU prior to any attempt at creating AV access. Many times, the veins are simply too deep to be palpated or visualized, even with a tourniquet in place. In one study, 53.5% had poor or absent veins on physical examination; however, with duplex ultrasound, adequate veins were found in 77% of this group of patients [24]. The forearm venous network is superficial and easily imaged with duplex ultrasound (see Figs. 31.5a–d). The preoperative vein mapping should visualize the entire length of the cephalic and basilic veins, evaluating diameter, continuity, distensibility, and presence of large branches.
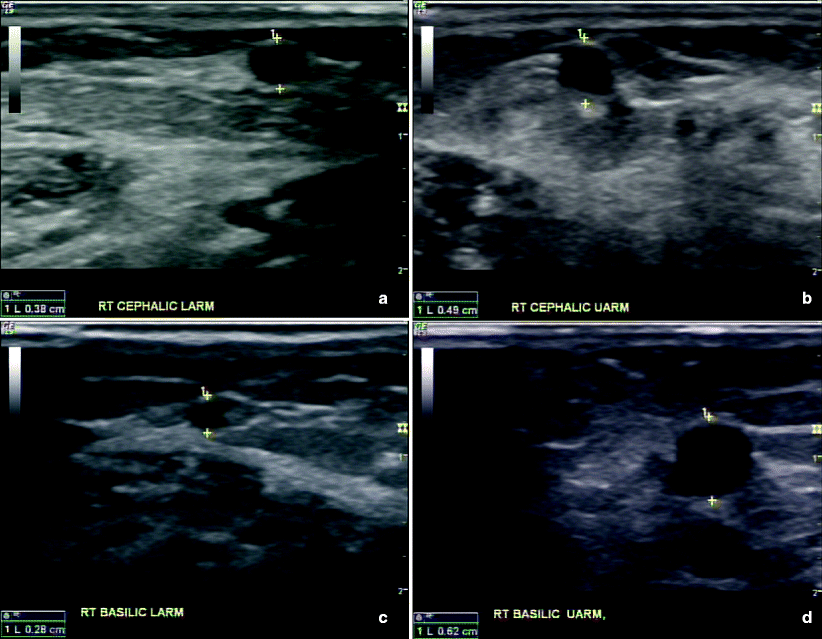

Fig. 31.5
(a–d) Preoperative ultrasound images of the right cephalic and basilic vein in the upper and lower arm
Preoperative vein mapping has been shown in numerous studies to increase the rate of autogenous AV access construction. Robbin et al. noted an increase in autogenous AV access placement from 32% to 58% after a preoperative DU program was started [25]. They also noted that preoperative vein mapping resulted in a change in the type and/or location of access surgery planned just after physical examination alone for 31% of their patients, including eight patients who received an autogenous rather than a prosthetic AV access [25].
Multiple studies have shown preoperative DU to predict successful AV access placement [26]. With preoperative DU, Silva et al. were able to demonstrate an increase in autogenous access placement from 14% to 63%, a decrease in early failure rate of the autogenous access from 38% to 8.3%, and a 1-year patency rate of 83% [21] when a venous luminal diameter of 2.5 mm or greater was used. Ascher also reported an increase of placement of autogenous AV access as well as an increase in the overall 1-year primary patency rate from 45% to 72% after implementation of a DU program [27]. Despite these promising results, there are additional studies that show that an increase in placement of autogenous AV access resulted in an increase of primary failure. A study by Miller et al. reported an early patency rate of only 47% and an 81% patency rate at 1 year [5]; this is likely due to the increased use of smaller caliber veins.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


