CHAPTER 81 Prosthetic Valve Endocarditis
Prosthetic valve endocarditis (PVE) is an uncommon but serious complication of heart valve replacement surgery. As the number of patients in the population with prosthetic valves continues to increase, the number of people at risk for PVE also rises. In fact, PVE now constitutes approximately 20% of all cases of endocarditis, up from only 1% to 5% during the past 2 decades.1 In most large series, the incidence of PVE is approximately 0.3% to 1% per patient-year, with a cumulative incidence of 3% at 5 years and 5% at 10 years.2 For unclear reasons, prosthetic valves are more likely to become infected in the aortic position than in the mitral position.3,4 This is in contradistinction to native valve endocarditis, wherein the mitral valve is more likely to become infected. In patients undergoing simultaneous aortic and mitral valve replacements, the incidence of prosthetic valve infection is greater, but the likelihoods of either prosthesis becoming infected are probably equal.5
HISTORICAL NOTE
In 1885, Osler observed an association between perioperative bacteremia and endocarditis.6 In 1935, Okell and Elliott noted that 11% of patients with poor oral hygiene had positive blood cultures for Streptococcus viridans, and that 60% of patients had bacteremia associated with dental extraction.7 Not long after the initial reports of valve replacements by Starr and Harken, the first reports of PVE appeared in the literature. Before the routine use of prophylactic antibiotics, Geraci and associates8 and Stein and coworkers9 reported incidences of early PVE of 10% and 12%, respectively. The use of routine prophylactic antibiotics was noted to reduce the incidence of early PVE to 0.2%.9 From the outset, the surgical management of PVE has been a formidable challenge. Particularly in the 1960s and 1970s, surgery for PVE was associated with an extremely high mortality rate. Discouraged by such early surgical experience, cardiac surgeons understandably made efforts to avoid operation for PVE. Although it was recognized that antibiotic treatment alone for PVE frequently produced fatal results, surgery for PVE was still reserved for the worst cases, and the surgical results were predictably poor. Hence, a vicious cycle developed in which surgery was avoided for fear of poor surgical outcomes, and poor surgical outcomes were achieved in very-high-risk cases.
In 1972, Ross successfully performed aortic root replacement for PVE using an aortic homograft.10 His report stressed the surgical principles still true today: complete surgical débridement of all infected tissue, the use of a homograft for reconstruction, and minimal use of foreign material in the infected area. In 1977, Olinger and Maloney11 reported replacement of an infected aortic prosthesis and external felt buttressing for correction of aorto-ventricular discontinuity. The following year, Frantz and colleagues12 reported repair of ventriculo-aortic discontinuity from endocarditis and abscess formation by aortic root replacement using a synthetic valved conduit. In 1974, Danielson and associates13 described a technique for treating extensive periannular abscess formation in native aortic valve endocarditis by translocation of a prosthetic aortic valve conduit into the ascending aorta and saphenous vein coronary artery bypass grafting. In 1981, Reitz and colleagues14 successfully applied this technique to treatment of prosthetic aortic valve endocarditis. In 1982, Symbas and colleagues15 combined aortic valve replacement with patch repair of periannular abscess cavity. In 1987, David and Feindel16 described techniques to reconstruct the mitral anulus with pericardium after débridement for PVE.
RISK
The risk of PVE to the patient is lifelong. However, as assessed by hazard function analysis, the risk of infection appears to be greatest during the first 3 months after valve implantation.17 Then it falls steadily, and by 12 months after implantation it remains at a constant low level.18 In the United States, approximately 20% of cases of PVE are diagnosed within the first 2 months after valve implantation.1 The incidence is approximately 1% during the first 12 postoperative months.4 After the first postoperative year, the incidence of PVE remains a constant low level of less than 1% per patient-year.2
By clinical convention, PVE is considered in two categories: early PVE and late PVE. This distinction was implemented because the prognosis for patients with PVE diagnosed soon after valve implantation was historically noted to be worse than if the diagnosis was made at a time remote from valve implantation. Although somewhat arbitrary, convention originally dictated that PVE diagnosed within 60 days of valve implantation was termed early PVE.19 However, the contemporary literature now variably defines the time point for early PVE as being within 2 months, 6 months, or 12 months after valve implantation.1,20 For many years, the incidence of PVE appeared to be evenly distributed between early and late.3 Recently, however, this demographic has changed, and the majority of cases of PVE are now considered “late.”1 The distinction between early and late PVE remains useful as it provides insight into the acquisition of the infection, the clinical course of the patient, and the management of the disease. One important distinction between early and late PVE is the bacteriology of the infection.
According to the definition provided by the Centers for Disease Control and Prevention, an infection involving an implanted prosthetic device within 12 months of an operative procedure is considered to be a nosocomial infection and is more likely to be acquired at the time of operation.21 Therefore, early PVE is often considered to arise from contamination of the valve during the perioperative period of valve implantation. However, a patient with an indwelling prosthetic valve placed more than 12 months before remains at risk for PVE resulting from health-care–associated infection.
Prosthetic valve endocarditis is considered to be a consequence of spontaneous or induced bacteremia.22 An association between nosocomial bacteremia and PVE was identified in the early 1990s by Fang and colleagues.23 Whether it occurs around the time of the valve implantation procedure or a different procedure, perioperative bacteremia is relatively common. Perhaps arising from infections such as wound or urinary infections, mediastinitis, or pneumonia, bacteremia increases the risk of prosthetic valve contamination. Likewise, bacteremia associated with procedures such as dental work, colonoscopy, prostate biopsy, and obstetrical procedures may cause PVE. Patients with indwelling urinary catheters24 and central lines are at particular risk for bacteremia; it is estimated that central line catheters cause at least 80,000 cases of central line–associated bacteremia annually.
Fortunately, bacteremia does not invariably cause PVE. Parker and colleagues25 reported that 32 of 890 patients had documented bacteremia in the early postoperative period after valve replacement. Surprisingly, of these 32 bacteremic patients, only 2 (6%) developed PVE, but both died. In a multi-institutional study review, Fang and colleagues23 determined that 18 of 115 patients (16%) with prosthetic heart valves developed PVE after having had bacteremia. In this study, the most common portals of bacterial entry were indwelling catheter infections (33%) and skin or wound infections (28%). Murray reported that the risk of developing endocarditis during an episode of hospital-acquired bacteremia is estimated at 1%.26 However, other authors have suggested the risk may be significantly higher.27
Fungal infections account for between 2% and 13% of cases of PVE and are usually lethal.4,28 Unfortunately, fungemia has become more common in hospitalized patients. Nasser and colleagues28 found that 9% of patients with prosthetic heart valves developed fungal endocarditis after documented candidemia. Of note, the mean time between candidemia and the clinical diagnosis of fungal PVE was 232 days. Hence, patients with candidemia must be aggressively treated in the acute setting and given careful long-term follow-up.
Implantation of a prosthetic valve in the setting of native valve contamination (but without evidence of active infection) may increase the risk of PVE. For that reason, many surgeons routinely culture excised valve leaflets to be certain the new valve is not contaminated at the time of implantation. Campbell and colleagues29 examined this practice and found that 32 or 222 patients (14%) who underwent elective valve replacement procedures had positive valve cultures. None of these patients had clinical evidence of infection. Only 1 of these 32 (3%) developed PVE. The authors concluded that most positive valve culture results were false positives. Nonetheless, PVE is such a significant complication that the practice of culturing excised valve tissue for unsuspected bacteria may well be justified. Patients with positive culture results should be treated.
Intraoperative contamination at the time of valve implantation is possible from a variety of sources. Cardiac surgical procedures are complex and entail numerous intravascular monitoring devices as well as the circuit of the heart-lung machine. Perhaps as a result of this complexity, the incidence of positive intraoperative blood cultures is high. Kluge and colleagues reported that 71% of cardiac surgical patients had positive intraoperative blood cultures.30 Several decades after this report from Kluge, the problem remains. In 1969, Ankeney and Parker31 reported that 19% of cardiac surgical patients had positive intraoperative blood cultures. In 2004, Shindo and colleagues32 reported that 16% of cardiac surgical patients who underwent cardiopulmonary bypass had positive intraoperative blood cultures.32
Intraoperative blood salvage via red blood cell recycling devices is routinely performed in cardiac surgical procedures. Of particular interest is the very high incidence of positive cultures in these blood-recycling circuits. Bland and colleagues33 reported that in 30 of 31 cases (97%), bags of recycled blood yielded positive cultures. Reents and colleagues34 reported that 90% of bags of recycled red blood cells had positive cultures. Shindo and colleagues32 reported that 67% of patients undergoing cardiovascular procedures with the use of recycled red blood cells but without the use of cardiopulmonary bypass had positive cultures from the recycled red blood cells.
Hemodialysis poses an additional risk for endocarditis, particularly as the number of people on chronic dialysis grows. Among patients with endocarditis, as many as 5% to 20% may be on chronic dialysis.1,35 Bacteremia is estimated to occur with hemodialysis at a frequency of one episode per 100 patient-months,36 primarily as a result of intravascular access (arteriovenous fistula or indwelling catheter). Furthermore, the prognosis of endocarditis among chronic dialysis patients is markedly worse than among nondialysis patients.37
Health care–associated infections are a significant source of PVE and may account for approximately 36% of cases of PVE.1 The majority of cases of health care–associated PVE are diagnosed in patients who were hospitalized for at least 48 hours before the diagnosis of PVE was made.1 Hence, it can be inferred that PVE was acquired after exposure to the health-care environment. The source of health care–associated PVE is frequently an intravascular device.1 The first year after valve implantation appears to be the time of highest risk for health care–associated PVE, with up to 70% of such cases diagnosed within this time frame.1
Prophylaxis
Since the mid 1950s, the American Heart Association (AHA) has made recommendations for antibiotic prophylaxis to attenuate the risk of endocarditis in patients undergoing dental procedures as well as other procedures with a risk of peri-procedure bacteremia. These recommendations have been regularly updated as data became available and have recently undergone a major revision.38
The most current AHA guidelines reflect an opinion that endocarditis prophylaxis for dental procedures should be reserved for patients with underlying cardiac conditions associated with the highest risk of adverse outcome from endocarditis.38 Acknowledging that the absolute risk of endocarditis from a dental procedure is unknown, the risk is currently estimated to be 1 in 14 million dental procedures. However, the risk in patients with a prosthetic heart valve is estimated to be 1 in 114,000 dental procedures. Hence, the AHA continues to recommend antibiotic prophylaxis for the prevention of PVE in these patients.38
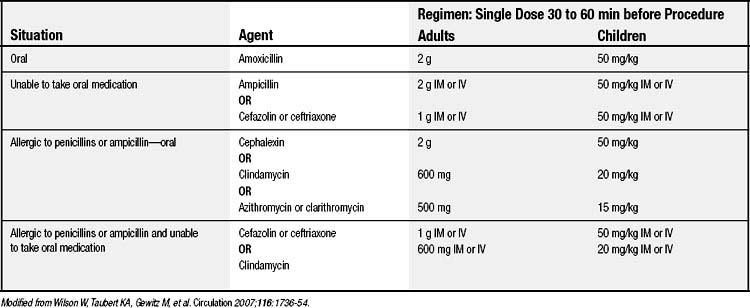
The antibiotic regimen recommended by the AHA for prophylaxis is listed in Table 81-1.
Type of Prosthesis
Most large series have found the incidence of PVE to be the same whether a mechanical or a bioprosthetic valve is used.39,40 However, mechanical valves appear to be at higher risk of infection within the first 3 months after implantation than bioprosthetic valves.41 Why the risk of early PVE should be somewhat higher with mechanical valves is unclear. Allografts lack prosthetic material and are believed to be relatively resistant to infection. Hence, the risk of endocarditis with allografts differs from that of either mechanical or bioprosthetic valves. Several series have demonstrated that the risk of endocarditis after allograft implantation is low and constant.42
The characteristics of the pathologic damage caused by the infection of PVE does tend to vary as a function of mechanical versus bioprosthetic valves. Because bacteria cannot adhere to the leaflets of mechanical valves in the absence of clot, the infection in mechanical valves becomes established at the junction of the sewing ring with the anulus. This results in abscess formation, valve dehiscence, and fistula formation.41,43,44 On the other hand, infection of bioprosthetic valves is commonly localized to the valve leaflets, leading to vegetations and leaflet perforation.41
Endocarditis after mitral valve repair is very rare, and its literature is scant. Gillinov,45 Gordon,4 and Karavas,46 along with their respective colleagues, reported the incidence to be well below 1%. The explanation most likely lies in the fact that mitral valve repair implants much less prosthetic material for potential infection than valve replacement.
Operation for Native Valve Endocarditis
Valve replacement in the setting of native valve endocarditis might logically be expected to increase the incidence of PVE. Surprisingly, many large studies have demonstrated the incidence to be approximately 4%, which is not higher than the overall reported incidence of PVE.24,47,48 On the other hand, several investigators have found patients to be at increased risk for PVE after valve replacement for native valve endocarditis. Ivert and colleagues found a fivefold increase in PVE when valve replacement was performed for active native valve endocarditis.17 Alexiou and colleagues49 reported a 6.7% incidence, and Wantanabe and colleagues50 reported an 11% incidence. In an extensive analysis, Moon and colleagues51 reported that the linearized rate of recurrent or residual endocarditis during the first 5 years after valve replacement for native endocarditis was 2.1% per patient-year. After the first 5 years, the rate fell to 0.9% per patient-year.
Historically, some investigators suggested that mechanical valves may pose a greater risk of recurrent infection than biological valves when placed in a patient with active native valve endocarditis. However, when they examined the situation critically, Moon and colleagues52 reported that the incidence of PVE did not differ for mechanical and biological valves placed for native valve endocarditis.
Use of an aortic allograft in native valve endocarditis offers the theoretical advantage of placing minimal foreign material in the setting of infection. In a series of 78 patients undergoing aortic valve replacement with an aortic allograft for native valve endocarditis, eight patients (10%) developed endocarditis in the allograft valve.53 McGiffin and colleagues42 reported a nonrandomized series that examined the influence of replacement valve type for active aortic endocarditis. In this series, the incidence of both early and late endocarditis in the replacement device was 5% (2 of 40 patients) when an allograft was used, and 10.8% (4 of 37 patients) when a bioprosthetic device was used. Okita and colleagues54 reported a 4.4% reinfection rate when an aortic allograft was used for aortic root replacement to treat severe periannular abscess.
Most authors recommend a mechanical or biological valve as a replacement in “simple” native valve endocarditis,52 which typically means endocarditis involving only the leaflets of the valve. In such circumstances, the available data suggest acceptably low rates of PVE in the replacement device. However, if significant periannular tissue destruction is found at operation for native aortic valve endocarditis, and particularly in the setting of Staphylococcus aureus infection, an allograft root replacement may be required. Nonetheless, Avierinos and colleagues55 recently reported that outcomes after aortic valve replacement for endocarditis with mechanical and bioprosthetic valves were not different from those done with allografts, even in cases involving periannular abscess formation.
MICROBIOLOGY
The microbiology of PVE is related to the etiology of the infection as well as to the time interval between valve implantation and infection. When diagnosed within the first 12 months after valve implantation, the infection is most commonly caused by Staphylococcus species. In prior years, the predominant cause of early infection was considered to be coagulase-negative staphylococci.4 More recently, S. aureus has been found in some series to be the most common cause of early PVE.1 Unlike staphylococcal species, streptococcal species are responsible for a small percentage of early PVE. Combined, streptococcal and diphtheroid species together account for less than 20% of early PVE.3 A variety of gram-negative bacteria and fungal infections account for most of the additional cases of early PVE.
On the other hand, the microbiology of PVE diagnosed beyond 12 months after implantation of the valve more closely resembles that of native valve endocarditis. This may reflect the fact that late PVE is acquired under conditions similar to native valve endocarditis. As in early PVE, the predominant organisms are gram-positive bacteria. However, in late PVE, streptococcal species are the most common, especially non–group D streptococci.22 Staphylococcal infections are also common, and in most series, infections caused by S. aureus are found less frequently than in early PVE.1 Thus, the bacteria seen in late PVE are typically more favorable to successful antibiotic treatment than those seen in early PVE (Table 81-2).
Table 81–2 Infectious Organisms in Prosthetic Valve Endocarditis (PVE)
| Organism | Early PVE (%) | Late PVE (%) |
|---|---|---|
| Staphylococcus aureus | 36 | 18 |
| Coagulase-negative staphylococci | 17 | 20 |
| Streptococcus species | 10 | 25 |
| Fungal species | 10 | 3 |
| Other | 27 | 34 |
Modified from Wang A, Athan E, Pappas PA, et al. JAMA 2007;297:1354-61.
PATHOLOGY
A feature common to all prosthetic valves is the sewing ring. This foreign material markedly reduces the inoculum of bacteria required to produce infection, so it frequently becomes the primary focus of infection. Thus, the pathologic hallmark of PVE is an abscess involving the valve sewing ring and the surrounding tissue. The incidence of paravalvular abscess in PVE is between 50% and 100%,56 and it occurs more commonly in the aortic position than in the mitral position.57 With abscess formation, the surrounding tissue may be progressively destroyed. With such destruction, valve dehiscence begins, and a paravalvular leak is produced. Likewise, the infection may destroy tissue between adjacent cardiac structures, creating a fistula. Extensive destruction of surrounding tissue may lead to pseudoaneurysm formation and even disruption of the aorta from the ventricle.
The infectious pathologies of mechanical and bioprosthetic valves may differ somewhat. Mechanical valve infection is virtually always focused in the sewing ring, and paravalvular abscess formation occurs in the vast majority of mechanical valve infections.39 On the other hand, infections of bioprosthetic valves may involve the leaflets alone, the sewing ring alone, or both. Unlike infection of mechanical valves, paravalvular abscess is therefore not a uniform finding in infected bioprosthetic valves. In some series, fewer than 20% of infected biologic valves had abscess formation.58 Infections of the bioprosthetic valve leaflets may also cause stenosis of the infected valve from vegetations, as well as valve leaflet destruction and perforation. Because the incidence of abscess formation is lower in tissue valve infection, success of antibiotic therapy alone has been reported to be greater in treatment of infected tissue valves.