Chapter 56 Prosthetic Graft Infection
Incidence
Prosthetic graft infection is relatively uncommon, with a reported incidence ranging from 0.2% to 5%. There are few prospectively obtained data analyzing the true incidence of prosthetic graft infection, but a survey of retrospective data with extended follow-up suggests that graft infections are influenced by the implant site, the indications for operation, and the host’s defense status, as manifested by the patient’s comorbid disease. A prospective, multicenter Canadian study of repair of unruptured abdominal aortic aneurysms revealed a graft infection rate of 0.2%.1 Szilagyi and colleagues’ analysis of 2145 patients undergoing a variety of vascular reconstructions, including aortofemoral, aortoiliac, and femoropopliteal reconstructions, revealed an overall graft infection rate of 1.5%.2 The insertion of a prosthetic graft in the femoropopliteal or femorotibial position has a higher rate of infection, ranging up to 5% in some reports.
Microbiology
Insight into the cause of vascular graft infections can be gained by examining the microorganisms most commonly recovered from infected grafts. Although any microorganism can potentially cause a graft infection, Staphylococcus aureus is the most prevalent bacterium.3 S. aureus is responsible for 25% to 50% of vascular graft infections, based on anatomic location. Over the last 15 to 20 years, Staphylococcus epidermidis has increasingly been identified as the responsible pathogen for a significant proportion of graft infections, predominantly the late-appearing, indolent type.
Graft infections are labeled as early (occurring less than 4 months after implantation) or late (occurring more than 4 months after implantation). Early-appearing graft infections are most commonly caused by more virulent organisms, including S. aureus. Coagulase-positive strains elaborate toxins that result in a vigorous host inflammatory response. Late-appearing graft infections are caused by less virulent gram-positive bacteria such as S. epidermidis. This organism is indolent and normally harbored in natural skin. It has the ability to adhere to prosthetic material and to secrete a glycocalyx biofilm that insulates the bacterium. Over time, this biofilm induces an inflammatory response that results in perigraft inflammation, which is seen clinically as perigraft fluid. This organism is fastidious in its attachment and is not easily isolated. S. epidermidis graft infections appear to have a sterile exudate, resulting in poor graft incorporation. Rarely do patients have a leukocytosis. This innocuous presentation has led many to view S. epidermidis infections as inflammatory allergic responses rather than bacterial graft infections.4 Bergamini and colleagues5 demonstrated that ultrasonication is required to physically separate S. epidermidis from the graft surface in order to culture it. In addition, broth culture yielded a higher rate of recovery than did agar plating. Bacterial recovery, which was only 30% when plated on agar media, rose to 72% in broth media. The combination of ultrasonication and broth culture resulted in a positive culture in 83% of cases in this report.
Other microorganisms such as fungi and gram-negative bacteria can also result in graft infections. Gram-negative bacteria such as Pseudomonas species, Escherichia coli, Enterobacter species, and Proteus species are highly virulent and result in dramatic clinical manifestations, such as anastomotic disruption and frank hemorrhage.6 These organisms are able to secrete potent proteases, which account for the tissue disruption that results in artery-graft disruption. Gram-negative infections also induce a substantial host inflammatory response that contributes to the disruption. Pseudomonas species infection is most commonly associated with graft disruption and hemorrhage. Severely immunosuppressed patients are also vulnerable to fungal graft infections, an otherwise rare clinical entity in the general population.
Cause and Pathophysiology
1. Intraoperative contamination
2. Hematogenous spread of bacteria
3. Direct contamination of graft by infection emanating from the skin, soft tissue, gastrointestinal tract, or genitourinary tract
Intraoperative Contamination
The patient’s endogenous flora from a site close to the prosthetic bed—especially from a colonized site, such as the skin or gut—is a common source of graft infection. Most of the intertriginous areas of the body, such as the axillae, groin, and interdigital spaces of the foot, contain large numbers of eccrine sweat glands and harbor large bacterial populations.7 Thus, prosthetic grafts placed in the groin have a well-characterized propensity to develop S. aureus infection. S. aureus bacteria from the groin area may contaminate only the wound, or they may also contaminate the graft surface if it is inadvertently dragged across the skin. The lymphatics in the groin may be contaminated at the time of surgery, especially if the patient has an open infected wound in an extremity. These lymphatics can also be a source of intraoperative graft infection. It is exceedingly rare for bacterial contamination to originate from the surgical team, from the graft, or from surgical instruments. The surgeon’s hands are seldom a major source of wound contamination.8
Bacteria can be present in diseased vessels or in the thrombus lining an aortic aneurysm.9 Van der Vliet and colleagues10 cultured the contents of the aneurysms of 216 patients and then followed the patients for more than 3 years. Positive cultures were found in 55 patients (25.5%); however, only 4 graft infections occurred (1.9%). Three of these four patients had positive cultures, but only two patients had the same organism as the original cultures. The authors concluded that bacteria in aneurysm contents have no demonstrable link to subsequent graft infections. A prospective study demonstrated that in patients undergoing peripheral vascular bypass surgery, 41% had positive arterial tissue cultures, 68% of which were coagulase-negative Staphylococcus.11 Subsequent graft infections were not reported; therefore the significance of the cultures is intriguing but unclear.
Hematogenous Spread
Seeding of an indwelling prosthesis by hematogenous spread of bacteria is potentially important; the frequency with which this occurs is unknown. In experimental animals, infusion of 10 million organisms of S. aureus immediately after implantation of a prosthetic aortic graft resulted in a 100% incidence of graft infection.12,13 Later studies demonstrated a lower incidence of graft infection with a longer time after implantation, suggesting that graft incorporation and the development of a pseudointima may provide some protection.14,15 Vulnerability to infection from bacteremia has been documented as late as 1 year after implantation, with 30% of aortic grafts becoming infected after a single bacterial infusion at that time.11 The anatomic difference between the infected and noninfected grafts was the presence of a complete neointimal lining. Parenteral antibiotics significantly reduced the incidence of graft infection, particularly when culture-directed therapy was applied to a remote infection. The significance of remote infection is unclear, but it suggests that transient bacteremia associated with, for example, dental procedures or colonoscopy may account for very late infections.
Prevention
The best way to avoid graft infection is to prevent it in the operating room and during the postoperative period. It has been shown that patients who are hospitalized for any length of time have an alteration in their cutaneous microflora.16 It is unclear whether this is caused by the underlying illness or by antibiotics. The preoperative stay, if any, should be as short as possible to avoid colonization with resistant bacteria. Prophylactic antibiotics have been shown to decrease the incidence of wound infections that could lead to graft infections. Most authors recommend the administration of a first-generation cephalosporin approximately 30 to 60 minutes before incision, with a scheduled dose continued for the first 24 hours. If the operation lasts an extended length of time, additional doses should be administered to maintain adequate circulating levels. There is no evidence to support the continuation of prophylactic antibiotics until indwelling lines, such as central venous or urinary bladder catheters, are removed. The prolonged use of postoperative antibiotics may even be detrimental, because the patient can develop antibiotic-associated colitis or other resistant-organism infections.
Meticulous surgical technique is important in preventing wound and graft infections. The graft must be handled carefully and should not be allowed to contact the patient’s skin. Adhesive drapes are commonly used to aid in preventing such contact, although there are no studies demonstrating a reduced incidence of wound or graft infection with the use of adhesive drapes, with or without iodine impregnation. If the adhesive plastic drape is separated from the skin during the operation, however, the infection rate increases.17,18 Simultaneous gastrointestinal procedures should be avoided to prevent intraoperative contamination. If an unplanned enterotomy occurs, graft implantation should be postponed, if possible. Dead space in surgical wounds should be minimized with the least possible amount of suture material. Irrigation of the peritoneal cavity is routine, but it can lead to washout of macrophages and opsonins. Irrigation should be used to remove all blood from the peritoneal cavity, because blood acts as an adjuvant for bacterial proliferation. If irrigation of a wound is performed, every effort should be made to evacuate the fluid.
In an effort to decrease the rates of graft and wound infections, certain authors have soaked gelatin-impregnated grafts with rifampin.19,20 This technique was associated with a decrease in the risk of groin wound infection, but showed no benefit in the rates of subsequent graft infection. Therefore, preoperative soaking of grafts in rifampin or other antibiotics is not indicated as a primary preventative measure.
Diagnosis
Clinical Presentation
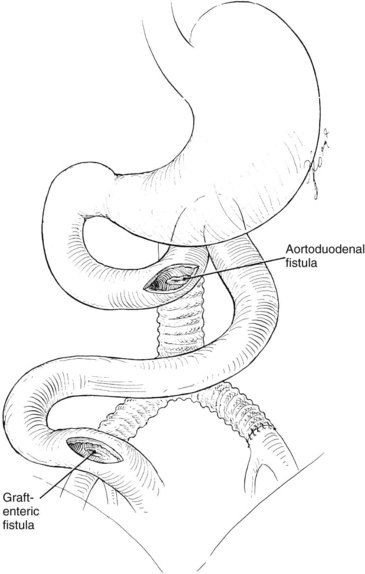
The clinical presentation of graft infection can be subtle and is influenced by the anatomic location of the graft. An infection of an infrainguinal graft frequently appears as cellulitis, soft tissue infection, drainage tract, or pseudoaneurysm. The clinical presentation of an extracavitary graft infection is usually not subtle. An intraabdominal graft infection may appear as systemic sepsis or as an ileus or abdominal distension, with or without tenderness. Occasionally, an aortic graft infection can result in an aortoenteric fistula, the first sign of which is a herald bleed. A patient with upper gastrointestinal bleeding and an aortic graft must be presumed to have an aortoenteric fistula until proved otherwise (Figure 56-1).
Imaging
Computed Tomography
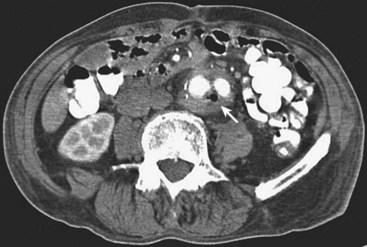
CT scanning is probably the most sensitive and reliable imaging test for the diagnosis of graft infections. Perigraft fluid collections, perigraft gas, anastomotic aneurysms, and distortion of tissue planes are all findings suggestive of graft infection and are all well visualized by a contrast-enhanced CT scan (Figure 56-2). Presence of gas or fluid around a graft more than 6 to 8 weeks after implantation is definitely abnormal and is presumptive evidence of graft infection.
O’Hara and coworkers published a study examining the natural history of periprosthetic air after abdominal aortic surgery.21 Twenty-six consecutive patients undergoing elective aortic aneurysm repair had CT scans performed at 3, 7, and 52 days postoperatively. Perigraft air was seen in 65% of patients in the first week following operation. All patients with periprosthetic air had spontaneous resolution, as demonstrated by late CT scanning obtained a mean of 52 days postoperatively (range, 21 to 85 days).
Magnetic Resonance Imaging
MRI is a useful modality for identifying perigraft fluid because of its ability to distinguish fluid from tissue by recognizing differences in signal intensity between T1- and T2-weighted images. The efficacy of this test was highlighted in a study by Olofsson and colleagues22 in which 18 patients suspected of having an aortic graft infection underwent preoperative MRI. Twelve patients also underwent CT scanning. MRI successfully identified 14 of 16 patients who were found to have an aortic graft infection at the time of operation, whereas CT was accurate in only 5 of 12 patients. It has been shown that there is only mild enhancement on T2-weighted images in grafts undergoing normal incorporation, whereas infected perigraft tissue demonstrates increased signal intensity.23 The role of MRI role in the diagnosis of aortic graft infections may become more prominent, but it is not routinely used for this purpose.
Angiography
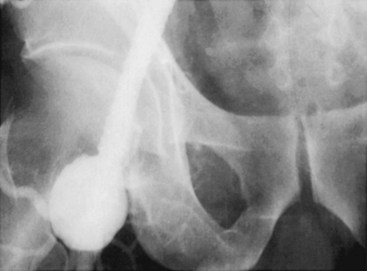
Angiography delineates intraluminal and extraluminal blood flow, but is unable to assess for extraluminal gas or fluid collections. It is therefore not useful for identifying graft infections, and CT and MRI are considered the tests of choice. Angiography can identify graft pseudoaneurysms (Figure 56-3), but these results are not diagnostic per se. Arteriography is used in this context not for the diagnosis of prosthetic graft infection, but to determine the options for arterial reconstruction. It is important to be careful in terms of timing the arteriogram with the CT scan, because both involve a contrast load; this is a consideration in a patient with renal insufficiency.
White Blood Cell Scanning
67Ga- or 111In-labeled white blood cell scanning is highly sensitive for graft infection. An 111In-labeled leukocyte or IgG scan is reliable and offers an advantage over 67Ga scanning because there is minimum nonspecific bowel uptake, resulting in a high target-to-background ratio and better results.24 111In-labeled leukocyte IgG scanning has been used by LaMuraglia and colleagues25 with impressive results. In 10 patients with positive scans, graft infection was confirmed at operation at the same site. Of the 15 patients with negative scans, the only one who had a false-negative result was a patient with an aortoduodenal fistula. The advantage of IgG scanning is that preparation is easier because the patient’s own blood is not required for the test and the tracer has a longer half-life.
Gastrointestinal Endoscopy
Endoscopy is a vital diagnostic tool in the evaluation of upper gastrointestinal tract bleeding.26 A patient with an aortic prosthesis and upper gastrointestinal bleeding must be suspected of having an aortoenteric fistula until proved otherwise. The initial test for this diagnosis is upper gastrointestinal endoscopy, which should be performed expeditiously once the diagnosis of aortoenteric fistula has been considered. The examination must be performed cautiously. The finding of an adherent blood clot in the duodenum confirms the diagnosis, and the patient should be taken immediately to the operating room. If endoscopy does not reveal any pathology, such as bleeding varices, gastritis, or a stomach or duodenal ulcer, an aortoenteric fistula may still be present but not seen. There are some who prefer to examine only the first and second portions of the duodenum; if this part of the study is negative, a presumptive diagnosis of aortoenteric fistula is made. Others prefer to use a smaller endoscope and actually examine the third and possibly the fourth portions of the duodenum; however, this requires endoscopic expertise and a low threshold for operative exploration should the study be equivocal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree