Diabetes mellitus (DM) accounts for >25% of all percutaneous coronary interventions. In patients with DM, drug-eluting stent implantation is associated with a reduced risk of restenosis and target lesion revascularization. However, concern has been raised about the incidence of late and very late stent thrombosis and the increased mortality rate, mostly after thienopyridine withdrawal. We evaluated the long-term prognostic effect of thienopyridine discontinuation after drug-eluting stent implantation on the subsequent occurrence of stent thrombosis and all-cause death among a cohort of high-risk “de novo” diabetic patients. From May 2002 to December 2005, 542 consecutive patients with DM underwent drug-eluting stent implantation at 2 hospitals in Milan, Italy. For study purposes, only the 217 patients who had not previously undergone percutaneous or surgical revascularization were considered in the final analysis. The follow-up time was curtailed at 3.5 years. Detailed information about dual antiplatelet therapy (DAT) were collected for all patients included. Of the 217 patients, 15 died (6.9%); in 9 cases, the cause of death was cardiac (4.1%). The incidence of cumulative stent thrombosis was 4.6% (10 patients); 3 stent thromboses were early (1.38%), 5 late (2.3%), and only 2 were very late (0.9%). Of the 10 cases of stent thrombosis, 5 were definite and 5 were probable. Most (80%) of the stent thromboses occurred within the first 6 months during DAT. The median duration of DAT was 420 days (interquartile range 350 to 859). DAT discontinuation was the only independent predictor of the follow-up events (hazard ratio 20.42, 95% confidence interval 4.99 to 83.62). In conclusion, DM remains an independent adverse factor on clinical outcome. In this setting, prolonged DAT, even beyond that recommended in the guidelines, might be beneficial.
Diabetes mellitus (DM) is universally regarded as a strong and independent risk factor for restenosis after balloon angioplasty and stent placement. Moreover, compared to those without DM, those with DM are 2 to 3 times more likely to experience a cardiovascular event. A growing body of epidemiologic evidence in this setting now supports the possibility that pathophysiologic alterations, such as insulin resistance, inflammatory signaling, endothelial dysfunction, and a pro-thrombotic state, drive both atherosclerosis and excessive neointimal formation after coronary intervention. Subgroup analyses that focused on patients with DM in randomized trials have shown that the use of drug-eluting stents (DESs) is associated with a reduced risk of restenosis and target lesion revascularization. However, very late stent thrombosis has emerged as a distinct entity overshadowing the use of DESs. Concerns have persisted regarding whether this phenomenon might compromise the long-term outcome after DES implantation, particularly after discontinuation of dual antiplatelet therapy (DAT). In the present observational study, we investigated the effect of DAT on stent thrombosis and all-cause death during a 3.5-year follow-up period in a small cohort of patients with DM and without any previous coronary revascularization before the index procedure.
Methods
The primary objective of the present study was to assess the composite incidence of stent thrombosis (definite and probable, see definitions 4 paragraphs below) and all-cause mortality during a 3.5-year follow-up period and its relation to DAT. The pattern of occurrence of stent thrombosis considering death as a competing risk was also of interest. We identified 542 consecutive patients with DM who had undergone successful implantation of sirolimus-eluting stents (Cypher, Cordis, Johnson & Johnson, Miami Lakes, Florida) or paclitaxel-eluting stents (Taxus, Boston Scientific, Natick, Massachusetts) from May 2002 to December 2005. For study purposes, we excluded 296 patients who had undergone previous coronary artery revascularization (percutaneous coronary intervention [PCI] or coronary artery bypass grafting), because in this setting, it would have been difficult to fully determine the duration of DAT and its relation to subsequent events. We also excluded another 29 patients because of missing or unreliable data about thienopyridine therapy. Thus, our study population included 217 patients.
The patients were treated at 2 hospitals in Milan, Italy (IRCCS San Raffaele and EMO-GVM Centro Cuore Columbus). The decision to implant a specific DES was at the discretion of the operator. Only patients with successful DES implantation, defined as <20% residual stenosis in the stented segment in the presence of grade 3 Thrombolysis In Myocardial Infarction flow, were considered in the present analysis. Patients who experienced an ST-segment elevation acute myocardial infarction (MI) <48 hours before the procedure were excluded, because it was not the practice of these institutions to implant a DES in patients with acute MI. All patients were recommended to begin ticlopidine or clopidogrel and aspirin ≥5 days before the procedure. The patients were usually telephoned before hospital admission and instructed to start taking DAT at home. For patients not pretreated with thienopyridines, a loading dose of 300 mg of clopidogrel was given on the day before the procedure. Aspirin was continued indefinitely and clopidogrel or ticlopidine for ≥3 months after sirolimus-eluting stent implantation and for ≥6 months after paclitaxel-eluting stent implantation. Glycoprotein IIb/IIIa inhibitor administration and the use of interventional devices were at the physician’s discretion. Standard qualitative and quantitative analyses and definitions were used for angiographic analysis. All patients provided written informed consent for the procedure and detailed data collection with approval from the local institutional review boards.
The follow-up period was curtailed at 3.5 years, when 70 patients were still at risk. The data were collected at 30 days and at 6-month intervals after the index procedure either at the scheduled clinical visits or by telephone interview. At the follow-up visit/interview, data were collected pertaining to the patients’ clinical status, antiplatelet drug therapy, and interim occurrence of any adverse events. Specifically, patients were asked whether they were taking aspirin, ticlopidine (Tiklid, Sanofi-Synthelabo S.p.A., Milan, Italy) or clopidogrel (Plavix, Sanofi-Synthelabo S.p.A.), how many tablets they were taking, and how long they had been taking them. If any antiplatelet medication had been discontinued, a detailed attempt was made to determine the point of discontinuation. In the case of doubt or uncertainty, the referring cardiologists or general practitioners were contacted for additional information.
The primary outcome was the combined end point of stent thrombosis and death. Stent thrombosis was classified as “early” when it occurred within 30 days after the procedure, “late” when it occurred between 30 days and 1 year after, and “very late” if it occurred after 1 year. Early stent thrombosis was defined as the occurrence of one of the following events: (1) angiographic documentation of complete or partial stent occlusion with thrombus and a target vessel-related acute clinical ischemic event; (2) autopsy documentation of complete or partial thrombotic stent occlusion; (3) MI in the distribution of the stented vessel; or (4) sudden cardiac death within 30 days.
Late and very late stent thrombosis were defined as the occurrence of one of the following events: (1) angiographic documentation of complete or partial thrombotic stent occlusion and a target vessel-related acute clinical ischemic event; (2) autopsy documentation of complete or partial thrombotic stent occlusion; or (3) MI in the distribution of the stented vessel in the absence of angiographic confirmation.
This definition of stent thrombosis corresponded to the definite and probable definition proposed by the Academic Research Consortium. Death was defined as all-cause mortality to fully analyze the effect of thienopyridines, which might be effective in ischemic heart disease but could be associated with an increased risk of bleeding and bleeding-related adverse events.
In the Results section, we have also reported on the incidence of other adverse events:
Cardiac death, defined as death from cardiac causes (including ischemic heart disease, heart failure, valvular heart disease) or any death from an unknown cause.
Major adverse cardiovascular events (MACE), defined as the occurrence of death, MI, or repeat revascularization, including target vessel revascularization and target lesion revascularization.
Multiple MACE, defined as the occurrence, for each patient, of ≥2 of the events included in the definition of MACE.
The aim of the present study was to investigate the effect of DAT discontinuation in patients with DM on the subsequent occurrence of stent thrombosis or death. Because discontinuation occurs during the follow-up period, each patient entered the study after de novo stent implantation and receiving treatment with thienopyridine. After the first discontinuation of the treatment regimen, the patient was switched to the group who had stopped the therapy until the end of the study. This was termed the internal time-dependent covariate and was used in a Cox model. The model estimated the hazard ratio between the patients who stopped the therapy and those who did not. This ratio was assumed to be constant in time (proportional hazard assumption). The proportional hazard for the treatment effect was evaluated using Schoenfeld residuals and included an interaction term between time and the time-dependent covariate.
As a first investigation of the role of each potential confounder, univariate Cox regression models were estimated. The variables “previous MI,” “insulin,” “bifurcation,” “lesion type,” “angina,” and “current smoker” were considered categorical variables and “age,” “creatinine,” “hemoglobin A1c,” “ejection fraction,” “lesion length,” “stent length,” “total stent length,” “maximum balloon diameter,” and “maximum inflation pressure” were considered continuous variables. For the latter, the possible presence of nonlinear effects was investigated by regression splines. A multivariate regression model with the treatment effect adjusted for the potential confounding effects of the other covariates was also considered. “Creatinine,” “hemoglobin A1c,” and “lesion length” were not included owing to the high number of missing values. To build a robust regression model, a backward variable selection according to the Akaike Information Criterion in multiple bootstrap samples was performed.
As the shape of the hazard function was not directly estimated by the Cox model, to evaluate the disease dynamic, a kernel-smoothed estimate of the hazard function from the Cox model, including the treatment effect, was obtained for the case of a patient who stopped the therapy from the beginning of the follow-up and for a patient who never stopped treatment.
The first event (ie, stent thrombosis, cardiac death, and noncardiac death) was considered the cause of treatment failure. Therefore, the probability of occurrence of each specific event as the first occurrence of treatment failure (crude cumulative incidence) was estimated using a nonparametric method for competing risk.
Statistical analyses were performed with R, version 2.7.2, for Windows (R Foundation for Statistical Computing, Vienna, Austria). All p values were 2-sided, and p ≤0.05 was considered statistically significant. We had full access to, and take full responsibility for, the integrity of the data; all have read and agreed to the manuscript as written.
Results
The baseline clinical and procedural characteristics of all patients are listed in Tables 1 and 2 . On average, all patients had a long stent length of 77.3 ± 54.8 mm implanted, suggesting the increased complexity of the study population. The median follow-up was 1,109 days (interquartile range 871 to 1,366). At 3.5 years of follow-up, MACE had occurred in 93 patients and multiple MACE in 13. Of the 271 patients, 15 had died; in 9 cases, the cause of death was cardiac. A total of 13 patients experienced a MI, but only 4 developed Q waves. Cumulative stent thrombosis occurred in 10 patients; it was early in 3, late in 5, and very late in only 2. Of the 10 cases of stent thrombosis, 5 were definite according to the Academic Research Consortium definition and 5 were probable. Of the patients with stent thrombosis, the case fatality rate was 40%. Both of the patients with very late stent thrombosis were no longer taking DAT at the event. The first patient had discontinued DAT after 1 year and stent thrombosis occurred at 781 days. The second patient had discontinued DAT at 90 days, and stent thrombosis occurred at 970 days. A total of 74 patients had undergone TVR, and 56 had undergone target lesion revascularization.
| Variable | Patients (n) | Mean ± SD | Median (Q1–Q3) | Range |
|---|---|---|---|---|
| Continuous | ||||
| Age (years) | 216 | 64.8 (9.7) | 65 (57–92) | 34–87 |
| Ejection fraction (%) | 211 | 51.6 (10.6) | 55 (48–60) | 20–80 |
| Hemoglobin A1c (%) | 128 | 7.3 (1.5) | 7 (6.3–8.1) | 1.01–11 |
| Serum creatinine (mg/dl) | 190 | 1.3 (1.1) | 1 (0.9–1.2) | 0–9.8 |
| Total cholesterol (mg/dl) | 142 | 166.6 (35.3) | 163.5 (142–182.5) | 100–368 |
| Low-density lipoprotein (mg/dl) | 107 | 91.4 (27.7) | 89 (73.9–104) | 49–250 |
| High-density lipoprotein (mg/dl) | 95 | 48.8 (11.5) | 48 (40–57) | 29–79 |
| Triglycerides (mg/dl) | 140 | 135.9 (68.9) | 121 (89–155.5) | 52–422 |
| Categorical | ||||
| Men | 173 (79.7%) | |||
| Family history for coronary artery disease | 83 (38.3%) | |||
| Hypertension | 161 (74.2%) | |||
| Current smoker | 24 (11.1%) | |||
| Unstable angina | 50 (23.0%) | |||
| Three-vessel disease | 92 (42.4%) | |||
| Insulin therapy ⁎ | 70 (32.4%) |
| Variable | Patients (n) | Mean ± SD | Median (Q1–Q3) | Range |
|---|---|---|---|---|
| Continuous | ||||
| Lesion length (mm) | 166 | 16.9 ± 11.4 | 14 (9–29.6) | 2–44.4 |
| Stent length/lesion (mm) | 216 | 33.1 ± 16.2 | 32 (24–33) | 8–116 |
| Total stent length/patient (mm) | 213 | 77.3 ± 54.8 | 59 (33–105) | 8–277 |
| Stents (n)/patient | 213 | 3.0 ± 2.1 | 2 (1–4) | 1–10 |
| Postprocedural minimum luminal diameter (mm) | 190 | 2.7 ± 0.5 | 2.7 (2.3–3) | 1.3–4.5 |
| Maximum balloon diameter (mm) | 210 | 2.9 ± 0.4 | 3 (2.8–3.5) | 0.1–4 |
| Maximum inflation pressure (atm) | 215 | 16.1 ± 4.3 | 16 (14–18) | 10–33 |
| Postprocedural creatinine (U/L) | 75 | 147.5 ± 166.8 | 90 (59.5–142) | 18–998 |
| Postprocedural creatinine-MB (ng/ml) | 63 | 12.4 ± 19.1 | 4.3 (2.4–14.2) | 0–93 |
| Other | ||||
| No. of stents/lesion | 216 | |||
| 1 | 176 (81%) | |||
| 2 | 32 (15%) | |||
| 3 | 6 (3%) | |||
| 4 | 2 (1%) | |||
| Left main disease | 10 (4.6%) | |||
| Glycoprotein IIb/IIIa inhibitor | 34 (15.7%) | |||
| Bifurcation treatment | 42 (19.4%) | |||
| Sirolimus-eluting stents | 132 (60.8%) | |||
| Type B2/C lesions ⁎ | 160 (76.6%) |
By study definition, information about DAT was available for all patients included. The median duration of DAT was 420 days (interquartile range 350 to 859) for all patients. The reasons for discontinuation are listed in Table 3 . DAT discontinuation depended mostly on thienopyridine nonadherence, with almost all patients compliant with aspirin (95.4%).
| Reason | Patients With Events ⁎ (n = 25) | Patients Without Events ⁎ (n = 192) |
|---|---|---|
| Bleeding | 1 (4.0%) | 5 (2.6%) |
| Concomitant anticoagulant therapy | 2 (8.0%) | 5 (2.6%) |
| End of prescribed therapy | 17 (68%) | 120 (62.5%) |
| Gastroenteritis | 1 (4.0%) | 0 |
| Hematologic disease | 0 | 1 (0.5%) |
| Poor adherence | 0 | 1 (0.5%) |
| Surgical procedure | 0 | 3 (1.5%) |
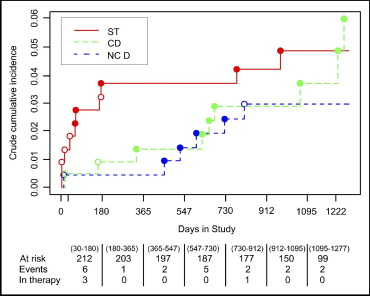
The crude cumulative incidence of stent thrombosis, cardiac death, and noncardiac death and the distribution of the events for patients who discontinued therapy and patients who did not by intervals are reported in Figure 1 . The estimate of the effect of stopping treatment, obtained from a univariate Cox model, was 13.7 (95% confidence interval [CI] 3.72 to 50.7). The effect was considered constant through time. The interaction between treatment and time was not statistically significant (p = 0.43). In a bootstrap resample procedure, the interaction was selected in 10% of the samples. The univariate Cox models for evaluating the effect of the different predictors on the composite risk of stent thrombosis and all-cause mortality are reported in Table 4 . Unstable angina was associated with adverse events during follow-up, significantly decreasing the event-free survival. A possible negative trend was noted for each 10 points of ejection fraction decrease and for renal dysfunction; however, no clear evidence was obtained for insulin treatment, final maximum balloon diameter, or inflation pressure. Even if longer lesions were treated and longer stents were implanted, compared to those in other studies, these variables did not significantly affect the long-term outcomes (hazard ratio [HR] 1.01, 95% CI 0.98 to 1.05, p = 0.46; and HR 1.01, 95% CI 0.99 to 1.03, p = 0.52 respectively), likely because they were a common characteristics for all patients included. In the development of the multivariate Cox model, creatinine, hemoglobin A1c, and lesion length were not included because of the high number of missing values (27, 89, and 51, respectively).