The prognostic value of an exaggerated exercise systolic blood pressure response (EESBPR) remains controversial. Our aim was to assess whether an EESBPR is associated with the long-term outcome in patients with diabetes mellitus and known or suspected coronary artery disease (CAD). From an initial population of 22,262 patients with known or suspected CAD who underwent treadmill exercise electrocardiography or exercise echocardiography at our institution, 2,591 patients with a history of diabetes mellitus were selected for the present study. EESBPR was defined as systolic blood pressure >220 mm Hg during exercise. The end points were all-cause mortality and hard events (ie, death or myocardial infarction). A total of 236 patients (9.1%) developed an EESBPR during the tests. During a mean follow-up of 6.5 ± 3.9 years, 484 patients died and 646 experienced hard events. The 10-year mortality rate was 16.6% in patients with an EESBPR compared to 30.9% in those without an EESBPR (p <0.001). The 10-year hard event rate was also lower in patients with an EESBPR (23.2% vs 38.9% in patients without an EESBPR; p <0.001). On multivariate analysis, an EESBPR remained independently associated with a lower risk of all-cause mortality (hazard ratio 0.53, 95% confidence interval 0.36 to 0.78, p = 0.001) and hard events (hazard ratio 0.57, 95% confidence interval 0.41 to 0.79; p <0.001). These results remained consistent in the subgroup of patients without a known history of CAD. In conclusion, an EESBPR was associated with improved survival and a lower rate of death or myocardial infarction in patients with diabetes mellitus and known or suspected CAD.
Hypertension at rest is a well-established risk factor for cardiovascular events, particularly in patients with diabetes mellitus (DM). The prognostic value of exercise hypertension, however, remains controversial. Although some studies have found an association of an exaggerated exercise blood pressure (BP) response with future hypertension and cardiovascular events in healthy subjects, studies evaluating patients with known or suspected coronary artery disease (CAD) have reported conflicting results. Furthermore, whether exercise hypertension provides prognostic information in patients with DM remains unexplored. Our aim was to assess the value of an exaggerated exercise systolic BP response (EESBPR) for predicting mortality and cardiac events in patients with DM and known or suspected CAD.
Methods
From June 1, 1994 to March 31, 2008, 22,262 patients aged ≥18 years with known or suspected CAD underwent symptom-limited treadmill exercise testing for clinical reasons at our institution. Of these patients, 2,956 had a history of DM. We excluded 326 patients who had received β blockers within 48 hours before the tests and 39 patients in whom the systolic BP failed to increase with exercise to greater than the at rest value. Thus, 2,591 patients were finally included in the present study. The demographic and clinical data and stress testing results were entered in our prospective database at the time of the procedures. DM was defined as a previous fasting serum glucose ≥126 mg/dl, nonfasting glucose of ≥200 mg/dl, use of antidiabetic drugs, or a self-reported diagnosis. Hypertension was defined as BP at rest of >130/80 mm Hg or a previously established diagnosis. Hypercholesterolemia was defined as a previously known low-density lipoprotein cholesterol level of ≥100 mg/dl, the use of lipid-lowering agents, or a self-reported diagnosis. Patients referred for evaluation of chest pain were classified as having typical angina, atypical/probable angina, or nonischemic chest pain. A history of CAD was defined as previous myocardial infarction (MI), previous coronary revascularization, or previous angiographic documentation of any ≥50% coronary stenosis. The electrocardiogram at rest was considered interpretable in the absence of left bundle branch block, pre-excitation, paced rhythm, left ventricular hypertrophy, repolarization abnormalities, or treatment with digoxin. All patients gave informed consent before testing, and the local research ethics committee approved the study.
The heart rate, BP, and a 12-lead electrocardiogram were obtained at baseline and at each stage of the exercise protocol. The patients were encouraged to perform a treadmill exercise test (Bruce protocol 93.9%, modified Bruce 3.2%, Naughton 1.5%, other protocols 1.5%) until they reached an end point. The exercise end points included physical exhaustion, severe angina, exercise-induced ST-segment deviation >2 mm, significant arrhythmia, or severe hypertension (systolic BP >240 mm Hg or diastolic BP >110 mm Hg). An EESBPR was defined as systolic BP >220 mm Hg during exercise. Significant ST-segment changes during the tests were defined as the development of ST-segment deviation of ≥1 mm, which was horizontal or sloping away from the isoelectric line 80 ms after the J point. A submaximal test was defined as the failure to achieve 85% of the maximum age-predicted heart rate.
A subset of 1,003 patients underwent treadmill exercise echocardiography; in these cases, the echocardiographic images were acquired at rest, at peak exercise, and immediately after exercise, as previously described. Echocardiographic ischemia was defined as the appearance of new or worsening wall motion abnormalities with exercise, except for worsening from akinesia to dyskinesia and isolated hypokinesia of the inferobasal segment.
Coronary angiography was performed at the discretion of the referring physician. Significant coronary stenosis was defined as a ≥50% lumen stenosis of the left main coronary artery or a ≥70% diameter stenosis of any other major epicardial coronary artery.
The follow-up data were obtained by review of hospital databases, medical records, death certificates, and telephone interviews. The main end points were all-cause mortality and hard events (ie, a composite of death or nonfatal MI). Percutaneous and surgical coronary revascularization procedures were also recorded.
Categorical variables are reported as percentages, and the comparison between groups was based on the chi-square test. Continuous variables are reported as mean ± SD, and the differences were assessed with the unpaired t test or Mann-Whitney U test, as appropriate. Survival free of the end point of interest was estimated using the Kaplan-Meier method, and the survival curves were compared using the log-rank test. The association of an EESBPR with the end points was assessed with Cox’s proportional hazard models. Hazard ratios with 95% confidence intervals were estimated. Statistical analysis was performed using the Statistical Package for Social Sciences software, version 15.0 (SPSS, Chicago, Illinois).
Results
The mean age was 63.8 ± 9.9 years, and 1,473 patients (56.9%) were men. Overall, 2,071 patients (80.2%) did not have a history of CAD. The demographic and clinical characteristics of the 2,591 patients are listed in Table 1 .
| Variable | All Patients (n = 2,591) | EESBPR | p Value | |
|---|---|---|---|---|
| No (n = 2,355) | Yes (n = 236) | |||
| Men | 1,473 (56.9%) | 1,341 (56.9%) | 132 (55.9%) | 0.77 |
| Age (years) | 63.8 ± 9.9 | 63.9 ± 9.9 | 61.9 ± 9.4 | 0.002 |
| Current smokers | 565 (21.8%) | 509 (21.6%) | 56 (23.7%) | 0.45 |
| Hypertension | 1,584 (61.1%) | 1,401 (59.5%) | 183 (77.5%) | <0.001 |
| Hypercholesterolemia | 1,391 (53.7%) | 1,248 (53.0%) | 143 (60.6%) | 0.03 |
| Family history of coronary artery disease | 320 (12.4%) | 273 (11.6%) | 47 (19.9%) | <0.001 |
| Previous myocardial infarction | 417 (16.1%) | 393 (16.7%) | 24 (10.2%) | 0.009 |
| ≤30 Days before testing | 171 (6.6%) | 167 (7.1%) | 4 (1.7%) | 0.001 |
| ≥30 Days before testing | 258 (10.0%) | 238 (10.1%) | 20 (8.5%) | 0.43 |
| Previous coronary revascularization | 218 (8.4%) | 209 (8.9%) | 9 (3.8%) | 0.008 |
| Percutaneous coronary intervention | 141 (5.4%) | 134 (5.7%) | 7 (3.0%) | 0.08 |
| Coronary artery bypass grafting | 87 (3.4%) | 85 (3.6%) | 2 (0.8%) | 0.02 |
| Atrial fibrillation | 79 (3.0%) | 78 (3.3%) | 1 (0.4%) | 0.01 |
| Left bundle branch block | 98 (3.8%) | 92 (3.9%) | 6 (2.5%) | 0.29 |
| Chest pain | 1,884 (72.7%) | 1,704 (72.4%) | 180 (76.3%) | 0.20 |
| Typical angina | 195 (7.5%) | 178 (7.6%) | 17 (7.2%) | 0.84 |
| Atypical/probable angina | 1,035 (39.9%) | 949 (40.3%) | 86 (36.4%) | 0.25 |
| Nonischemic chest pain | 654 (25.2%) | 577 (24.5%) | 77 (32.6%) | 0.006 |
| Medication | ||||
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 885 (34.2%) | 804 (34.1%) | 81 (34.3%) | 0.96 |
| Calcium channel blockers | 440 (17.0%) | 400 (17.0%) | 40 (16.9%) | 0.99 |
| Nitrates | 681 (26.3%) | 626 (26.6%) | 55 (23.3%) | 0.28 |
| Diuretics | 233 (9.0%) | 218 (9.3%) | 15 (6.4%) | 0.14 |
A total of 236 patients (9.1%) developed an EESBPR during the tests. The patients with an EESBPR were slightly younger and had a greater prevalence of cardiovascular risk factors compared to those not developing an EESBPR. However, the former had a lower likelihood of a history of CAD. There were no significant differences regarding antihypertensive medications between the 2 groups ( Table 1 ). The exercise data are listed in Table 2 .
| Variable | All Patients (n = 2,591) | EESBPR | p Value | |
|---|---|---|---|---|
| No (n = 2,355) | Yes (n = 236) | |||
| Resting systolic blood pressure (mm Hg) | 139.1 ± 20.6 | 137.5 ± 19.6 | 165.8 ± 18.9 | <0.001 |
| Peak systolic blood pressure (mm Hg) | 178.2 ± 32.0 | 172.0 ± 26.6 | 238.7 ± 10.1 | <0.001 |
| Resting heart rate (beats/min) | 81.2 ± 15.5 | 81.3 ± 15.7 | 81.0 ± 12.0 | 0.89 |
| Peak heart rate (beats/min) | 143 ± 21 | 142.5 ± 20.8 | 145.5 ± 17.7 | 0.01 |
| % of maximum age-predicted heart rate | 91.3 ± 12.3 | 91.2 ± 12.4 | 92.0 ± 10.9 | 0.30 |
| Rate-pressure product (×10 3 mm Hg beats/min) | 25.5 ± 6.3 | 24.6 ± 5.7 | 34.7 ± 4.4 | <0.001 |
| Sub-maximal test | 686 (26.5%) | 641 (27.2%) | 45 (19.1%) | 0.007 |
| Metabolic equivalents (METs) | 8.1 ± 3.2 | 8.1 ± 2.9 | 8.0 ± 2.5 | 0.57 |
Of the subgroup of 2,285 patients with interpretable electrocardiograms at rest, 470 (20.6%) developed significant exercise-induced ST-segment abnormalities, and the latter occurred less frequently in patients developing an EESBPR (13.2% vs 21.4% in patients without an EESBPR, p = 0.004). In contrast, of the subgroup of 1,003 patients undergoing exercise echocardiography, 419 (41.8%) developed new or worsening wall motion abnormalities during exercise. These were also less likely in patients developing an EESBPR (21.1% vs 43.0% in patients who did not develop an EESBPR, p = 0.001). The left ventricular ejection fraction increased from 55.5 ± 10.3% to 57.3 ± 14.9% in patients without an EESBPR and from 59.9 ± 7.2% to 64.3 ± 11.7% in those with an EESBPR.
A total of 357 patients underwent coronary angiography within 90 days after the treadmill tests. Of them, 288 (80.7%) had significant coronary stenoses. The likelihood of significant coronary stenoses in patients with an EESBPR who had undergone coronary angiography (72.2%) was not significantly different from that of the patients without an EESBPR (81.1%, p = 0.35).
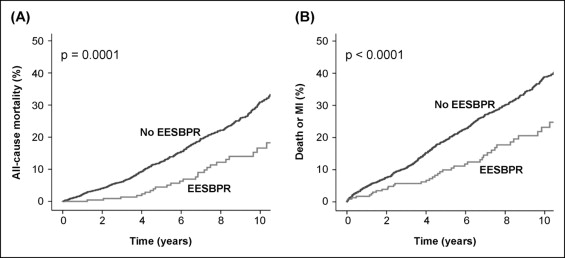
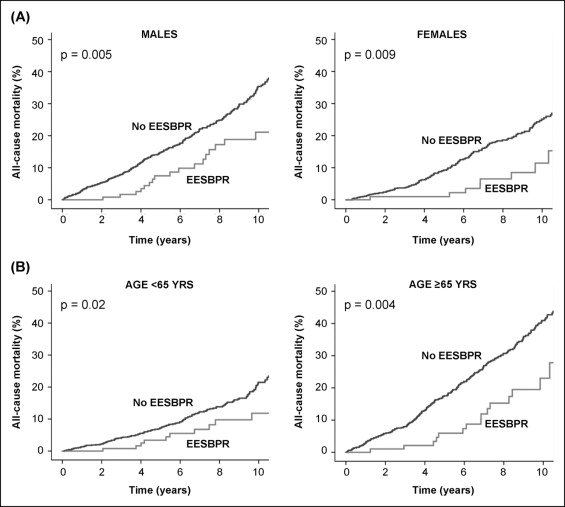
During a mean follow-up of 6.0 ± 3.7 years, 484 patients died, 286 experienced MI, 309 underwent percutaneous coronary intervention, and 206 underwent coronary artery bypass surgery. A total of 646 patients experienced a hard event. The 10-year mortality rate was 16.6% in patients with an EESBPR compared to 30.9% in patients without an EESBPR (p = 0.0001; Figure 1 ). These results remained consistent when the patients were stratified according to gender and age ( Figure 2 ). The 10-year hard event rate was also lower in patients with an EESBPR (23.2% vs 38.9% in patients without an EESBPR, p <0.0001; Figure 1 ). On multivariate analysis, EESBPR was independently associated with a lower risk of all-cause mortality and hard events ( Table 3 ).