To assess the effect of autologous bone-marrow cell (BMC) therapy in patients with acute myocardial infarction in a rigorous double-blind, randomized, placebo-controlled trial. Patients with reperfusion >6 hours after symptom onset were randomly assigned in a 2:1 ratio to receive intracoronary BMC or placebo therapy 5 to 7 days after symptom onset. The patients were stratified according to age, acute myocardial infarction localization, and left ventricular (LV) function. Rigorous double-blinding was ensured using autologous erythrocytes for the placebo preparation that was visually indistinguishable from the active treatment. Serial cardiac magnetic resonance imaging studies were performed before study therapy and after 1, 3, and 6 months. The primary end point was the difference in the LV ejection fraction from baseline to 6 months. The secondary end points included changes in the LV end-diastolic and end-systolic volume indexes and infarct size. A total of 42 patients were enrolled (29 in the BMC group and 13 in the placebo group) in the integrated pilot phase. A mean of 381 × 10 6 mononuclear BMCs were administered. The baseline clinical and cardiac magnetic resonance imaging parameters did not differ. Compared to baseline, the difference in LV ejection fraction for the placebo group versus BMC group was 1.7 ± 6.4% versus −0.9 ± 5.5% at 1 month, 3.1 ± 6.0% versus 1.9 ± 4.3% at 3 months, and 5.7 ± 8.4% versus 1.8 ± 5.3% at 6 months (primary end point; not significant). No difference was found in the secondary end points between the 2 groups, including changes in infarct size or LV end-diastolic and end-systolic volume indexes. In conclusion, in this rigorous double-blind, randomized, placebo-controlled trial, we did not observe an evidence for a positive effect for intracoronary BMC versus placebo therapy with respect to LV ejection fraction, LV volume indexes, or infarct size.
Conflicting results for intracoronary bone marrow cell (BMC) therapy for improvement of left ventricular (LV) ejection fraction (EF) in patients with acute myocardial infarction (AMI) have been reported. In the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI), the LVEF improved significantly more in the BMC-treated patients compared to the placebo group. However, in other randomized trials, this could not be shown or was not maintained during follow-up. A randomized double-blind setting—often ethically rejected because of BMC aspiration and intracoronary placebo administration—has been implemented in 3 trials. Despite the double-blind setting, syringes for BMC or placebo therapy could be easily differentiated because of the non–cell-based placebo preparation. Using autologous erythrocytes for the placebo preparation, double blinding can be completely ensured. Different techniques for the evaluation of LVEF have been used, including echocardiography, single photon emission computed tomography, levocardiography, and cardiac magnetic resonance imaging (CMRI). Short-axis volumetry using CMRI is the reference standard for the assessment of LV volumes and LVEF. From previous trials, a high number of injected cells and application time of >5 days after AMI have been linked to improved outcomes in BMC-treated patients. Against this background, we performed a randomized, placebo-controlled, rigorous, double-blind study—the intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) study—including the intracoronary administration of a high cell number at days 5 to 7 after AMI with 4 serial CMRI studies within 6 months to determine whether BMC therapy is superior to placebo regarding improvement in the LVEF.
Methods
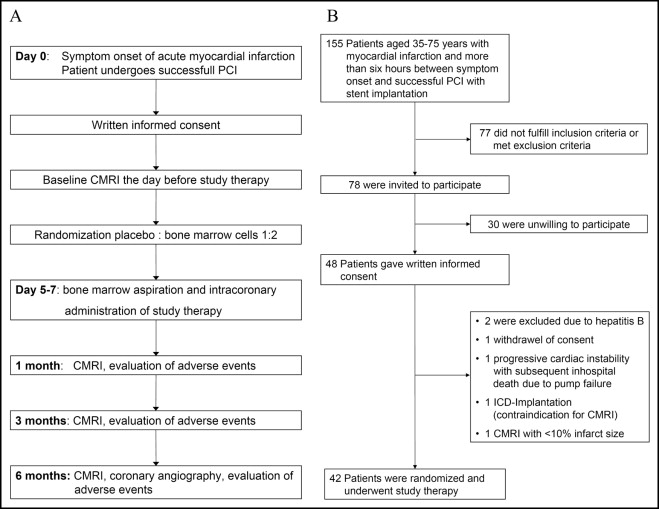
We screened patients aged 35 to 75 years with AMI. The inclusion criteria were AMI with 6 to 48 hours between symptom onset to successful percutaneous coronary intervention, a proximal lesion, creatine kinase level >1,000 U/L, and infarct size of >10% of the LV muscle mass, as determined by CMRI. The exclusion criteria were a history of myocardial infarction, cardiogenic shock, contraindications to CMRI or 12 months of dual antiplatelet therapy, a history of hematologic disease, chemotherapy, previous stem cell therapy or treatment with granulocyte colony-stimulating factor, severe renal dysfunction, or documented terminal illness. The study protocol conformed to the Declaration of Helsinki and was approved by the local ethics committee, Federal Office for Radiation Protection, and the Paul-Ehrlich Institute (the ClinicalTrials.gov number is NCT00669227 ). All patients gave written informed consent. The study design is shown in Figure 1 . The patients were randomly assigned in a 2:1 ratio to either the mononuclear BMC group or the placebo group by external randomization. The patients were stratified according to following criteria: age <60 or ≥60 years, anterior versus nonanterior AMI, and a reduction in LVEF (severe vs nonsevere) as visually assessed by echocardiography the day after percutaneous coronary intervention. Baseline CMRI was done the day before study therapy. All patients underwent BMC aspiration and intracoronary administration of the study therapy. CMRI and clinical evaluation for adverse events were repeated at 1, 3, and 6 months after AMI, including coronary angiography after 6 months.
With the patient under local anesthesia, the bone marrow was aspirated from the iliac crest into 20-ml syringes containing 500 IU heparin, 0.04 mg gentamicin, and 3,000 IU penicillin in 3-ml 0.9% sodium chloride. The aspirate was shipped at room temperature between the catheter laboratory and cell-processing laboratory within about 15 minutes. The cells were morphologically checked to exclude any subclinical hematologic disease. Mononuclear cells were isolated with Ficoll density gradient centrifugation (Cambrex, Belgium, same charge as in REPAIR-AMI, 823 g, 20 minutes, without a brake), washed, and resuspended in 15 ml 0.9% sodium chloride with 2% human albumin. The placebo syringes also contained 15 ml 0.9% sodium chloride with 2% human albumin and autologous erythrocytes with a hematocrit of 0.1% without BMC. The placebo and active treatment could not be visually distinguished, ensuring rigorous double-blinding. The cell preparations were analyzed using a fluorescence-activated cell sorter. For the identification of populations with the dual platform lysis and wash method, we used directly conjugated antibodies against human CD34, CD45 (BD Biosciences Pharmingen, San Diego, California), CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany), and vascular endothelial growth factor-R2 (R&D Systems, Minneapolis, Minnesota). The analyses were performed according to the protocol of the International Society of Hematotherapy and Graft Engineering. Viability testing was performed using the trypan blue method. Hematopoietic colony assays (burst-forming unit-erythroid and colony-forming unit granulocyte, erythrocyte, monocyte, megakaryocyte) and mesenchymal colony assays (colony-forming unit fibroblast) were performed using Methocult and MesenCult (StemCell Technologies, Cologne, Germany) according to the manufacturer’s instructions. The colony-forming units were counted after 14 days. The reproducibility of the hematopoietic colony assays was verified by regular, successful participation in a global proficiency testing program (Stem Cell Technologies). Patients underwent serologic testing for hepatitis and human immunodeficiency virus infection. The study therapy was administered the same day of bone marrow aspiration using the stop-flow technique through an over-the-wire balloon catheter positioned within the stented segment.
CMRI was done using a 1.5 T Intera CV whole body magnetic resonance scanner (Philips Medical Systems, Best, The Netherlands). All data were acquired using a dedicated 5-element cardiac phased-array coil. Parallel imaging was used for all scans. The LV volumes, LVEF, and LV muscle mass were analyzed using a ViewForum workstation using short-axis volumetry, as described previously. To determine the LV function, a retrospective electrocardiographic-gated segmented k-space balanced turbo-field-echo sequence (steady-state-free-precession) without view sharing was used in short-axis views perpendicular to the true heart axis. The slice thickness was 10 mm without a gap. For whole coverage of the right and left ventricles, the slice number was individually adjusted. Depending on the required field of view, the spatial resolution was between 1.7 × 1.8 mm and 2.3 × 1.8 mm in-plane. The following parameters were used: echo time 1.7 ms, repetition time 3.4 ms, parallel imaging (sensitivity encoding) factor 2. Papillary muscles were assigned to the myocardium. A late enhancement study was performed 15 minutes after infusion of 0.2 mmol/kg body weight gadolinium-diethylenetriaminepentaacetate (Magnevist, Schering, Germany) using a 3-dimensional spoiled turbo gradient echo sequence with a selective 180° inversion recovery prepulse in the short axis covering the whole left ventricle (20 to 22 5-mm slices). The infarct size was defined as late enhancement in relation to the LV muscle mass. Late enhancement was quantitatively assessed on a ViewForum Workstation. The analyses were done by 2 physicians with consensus reading.
The primary end point was the difference in the LVEF from baseline to 6 months, as measured by CMRI. The study was designed with 80% power to detect a significant difference in LVEF at a 1-sided significance level of 2.5%. A clinically important difference was defined as an absolute LVEF difference of 2.5% between the 2 treatment groups. On the basis of a difference in LVEF of 5.0 ± 7.7% in a subgroup of patients fulfilling the inclusion and exclusion criteria derived from a previously reported population, we hypothesized a LVEF difference in the control arm of 5.0% with a SD of 7.5%. From several reports of BMC therapy, we hypothesized a 7.5% increase in LVEF in the BMC population. Because the CMRI data for the difference in LVEF were limited during the period of study planning, an interim analysis after inclusion of 42 patients was integrated to allow a valid calculation of the final sample size. With a 2:1 randomization in favor of BMC therapy, we calculated a total sample size of 312 patients. According to the interim analysis, 2 stopping rules were used. First, if active treatment were superior to placebo, with p <0.01; and second, if no basis for improvement of LVEF by active treatment therapy was found, with p >0.5. Randomization according to the stratifying criteria was performed by an external institute not involved in patient recruitment, study therapy preparation, or the follow-up visits. Secondary CMRI end points included changes in LV end-diastolic and end-systolic volume indexes and infarct size. The clinical end points included major adverse cardiac events, defined as death, myocardial infarction recurrence, and rehospitalization for heart failure. Repeated myocardial infarction was defined as creatine kinase elevation >2 times the upper normal limit with a significant MB fraction (>6%) or new Q-waves on the electrocardiogram. Rehospitalization because of heart failure was defined as hospitalization with typical clinical findings of heart failure requiring a change in medication. The definition for hypertension and hyperlipidemia have been previously published. Stent thrombosis was defined according to the Academic Research Consortium criteria.
The categorical variables were compared using the chi-square test. Variables approximated a normal distribution, and the Brown and Forsythe test confirmed homogeneity of the variance between the 2 groups. Values are presented as the mean ± SD. t Tests and analysis of variance were performed. p Values <0.05 were considered statistically significant.
Results
During the enrollment period, 155 patients, aged 35 to 75 years, with AMI successfully reperfused >6 hours after symptom onset by stent implantation, were screened ( Figure 1 ). A total of 42 patients were randomized, underwent bone marrow aspiration and received the study therapy. The median delay to percutaneous coronary intervention from symptom onset was 14.3 hours. Of the 42 patients, 29 were randomly assigned to receive BMC therapy and 13 to receive placebo. Both groups were well-matched with respect to the baseline clinical data and procedural parameters ( Table 1 ).
| Variable | Placebo Group (n = 13) | BMC Group (n = 29) | p Value |
|---|---|---|---|
| Age (years) | 61.1 ± 9.3 | 61.0 ± 8.1 | 0.96 |
| Gender | |||
| Female | 5 (38%) | 3 (10%) | 0.04 |
| Male | 8 (62%) | 26 (90%) | |
| Hypertension | 9 (69%) | 16 (55%) | 0.39 |
| Hyperlipidemia | 9 (69%) | 22 (76%) | 0.65 |
| History of smoking | 7 (54%) | 18 (61%) | 0.62 |
| Diabetes mellitus | 5 (38%) | 9 (31%) | 0.64 |
| Insulin required | 1 (8%) | 2 (7%) | 0.93 |
| Body mass index (kg/m 2 ) | 27.9 ± 4.4 | 28.5 ± 3.5 | 0.63 |
| Cardiogenic shock | 1 (8%) | 0 (0%) | 0.13 |
| Cardiopulmonary resuscitation | 1 (8%) | 2 (7%) | 0.93 |
| Stratifying criteria | |||
| Age | 0.90 | ||
| <60 years | 6 (46%) | 14 (48%) | |
| ≥60 years | 7 (54%) | 15 (52%) | |
| Infarct location | 0.74 | ||
| Anterior wall | 7 (54%) | 14 (48%) | |
| Nonanterior wall | 6 (46%) | 15 (52%) | |
| Reduction in left ventricular function | 0.86 | ||
| Severe | 5 (38%) | 12 (41%) | |
| Nonsevere | 8 (62%) | 17 (59%) | |
| Procedural characteristics | |||
| Drug-eluting stent | 4 (31%) | 8 (28%) | 0.83 |
| Stent diameter (mm) | 3.1 ± 0.4 | 3.2 ± 0.6 | 0.70 |
| Glycoprotein IIb/IIIa inhibitor | 12 (92%) | 26 (90%) | 0.79 |
| Stents implanted | 1.5 ± 0.7 | 1.8 ± 1.2 | 0.40 |
| Length of stented segment (mm) | 33.5 ± 18.9 | 33.3 ± 18.0 | 0.97 |
| Maximum inflation pressure (atm) | 16.9 ± 2.7 | 17.7 ± 3.4 | 0.45 |
| Maximum creatine kinase (U/L) | 2,992 ± 2051 | 3,543 ± 2,248 | 0.46 |
| Interval from symptom onset to first balloon dilation (hours) | 0.21 | ||
| Mean | 15.9 ± 9.0 | 20.8 ± 12.3 | |
| Median | 13.0 | 18.7 | |
| Interval from bone marrow cell aspiration to infusion of study therapy (hours) | 0.78 | ||
| Mean | 6.3 ± 0.8 | 6.2 ± 1.5 | |
| Median | 6.1 | 6.1 | |
| Interval from symptom onset to infusion of study therapy (days) | 0.80 | ||
| Mean | 6.6 ± 1.5 | 6.8 ± 2.5 | |
| Median | 6.6 | 6.0 | |
| Interval from baseline CMRI to 1-month CMRI (days) | 0.68 | ||
| Mean | 41 ± 8 | 40 ± 8 | |
| Median | 39 | 40 | |
| Interval from 1-month CMRI to 3-month CMRI (days) | 0.65 | ||
| Mean | 59 ± 10 | 58 ± 8 | |
| Median | 62 | 57 | |
| Interval from 3-month CMRI to 6-month CMRI (days) | 0.26 | ||
| Mean | 98 ± 25 | 91 ± 13 | |
| Median | 91 | 91 |
A total of 128 ± 14 ml (median 130, interquartile range 124 to 136) of bone marrow was aspirated. The cell morphology was normal. Intracoronary injection of the study therapy was performed a median of 6.1 days (interquartile range 5.5 to 7.3) after the onset of AMI and a median of 6.1 hours (interquartile range 5.7 to 6.9) after BMC aspiration. In the BMC group, a mean of 381 × 10 6 mononuclear BMCs were administered. The cell characteristics are listed in Table 2 . Drug therapy did not differ between the placebo and BMC groups at hospital discharge or at 1, 3, and 6 months of follow-up ( Figure 2 ).
| Variable | Mean ± SD | Median (IQR) | Range |
|---|---|---|---|
| No. and viability | |||
| No. of administered mononuclear cells (×10 6 ) | 381 ± 130 | 324 (284; 458) | 227–629 |
| Viability of cells (%) | 99 ± 1.2 | 100 (99; 100) | 95–100 |
| Surface markers (fluorescence-activated cell sorter analyses) | |||
| CD34+ CD45+ | |||
| % | 0.8 ± 0.3 | 0.7 (0.5; 1.0) | 0.3–1.6 |
| Absolute cell number × 10 6 | 3.2 ± 1.9 | 2.7 (1.9; 3.8) | 0.6–8.4 |
| CD133+ CD45+ | |||
| % | 0.4 ± 0.2 | 0.4 (0.2; 0.6) | 0.1–1.0 |
| Absolute cell number × 10 6 | 1.6 ± 1.1 | 1.5 (0.7; 2.1) | 0.2–5.3 |
| CD34+, CD133+, CD45+ | |||
| % | 0.4 ± 0.3 | 0.3 (0.2; 0.5) | 0.1–1.1 |
| Absolute cell number × 10 6 | 1.6 ± 1.1 | 1.4 (0.8; 1.9) | 0.2–5.5 |
| Vascular endothelial growth factor-R2+, CD45+ | |||
| % | 0.2 ± 0.1 | 0.1 (0.1; 0.2) | 0.1–0.5 |
| Absolute cell number × 10 6 | 0.6 ± 0.4 | 0.4 (0.3; 0.7) | 0.2–2.0 |
| Vascular endothelial growth factor-R2+, CD34+, CD45+ | |||
| % | 0.1 ± 0.1 | 0.1 (0.1–0.1) | 0.02–0.3 |
| Absolute cell number × 10 6 | 0.3 ± 0.2 | 0.2 (0.2–0.3) | 0.1–1.1 |
| Colony-forming unit capacity | |||
| Burst-forming unit-erythroid (n/10 6 cells) | 1,815 ± 808 | 1,750 (1,313; 2,250) | 533–4,875 |
| Colony-forming unit = granulocyte, erythrocyte, monocyte, megakaryocyte (n/10 6 cells) | 360 ± 160 | 319 (200; 500) | 67–688 |
| Colony forming unit fibroblast (n/10 6 cells) | 17 ± 7 | 16 (11–21) | 5–35 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


