Circulating nonesterified or free fatty acids (FFAs) may contribute to the development of cardiovascular pathology and correlate with ischemia in acute cardiovascular conditions. The aim of this study was to assess whether serum levels of FFAs are associated with long-term prognosis in subjects with stable coronary heart disease. This observational prospective cohort study included 1,206 participants in 3-weeks inpatient rehabilitation programs after acute myocardial infarction, coronary syndromes, or coronary intervention at 2 rehabilitation clinics in Germany (1999 to 2000). Eight-year prognosis (time to a secondary fatal or nonfatal cardiovascular disease event including myocardial infarction and stroke [n = 153] and time to death from any cause [n = 124]) was examined according to FFA quartiles and in spline regression. FFAs were correlated with established serum markers of cardiovascular risk and strongly related to secondary cardiovascular events and all-cause mortality in age- and gender-adjusted analysis. When additionally controlling for multiple established risk factors and risk markers, the hazard ratio in the fourth versus first quartile was 1.34 (95% confidence interval 0.79 to 2.24) for secondary cardiovascular events and 1.09 (95% confidence interval 0.62 to 1.91) for all-cause mortality. Dose-response modeling suggested that very high FFAs might predict an increased risk for mortality (hazard ratio 1.98, 95% confidence interval 0.98 to 4.02, for 95th percentile vs first quartile). In conclusion, FFAs are closely correlated with cardiovascular risk markers, and in particular, very high FFA might identify patients with stable coronary heart disease with worse prognoses.
Levels of circulating free fatty acids (FFAs), which generally are known as important substrates for energy homeostasis, have more recently attracted attention as potential risk factors for cardiovascular disorders. Increased levels have been found in obesity, and prospective associations with insulin resistance and type 2 diabetes mellitus have been described. Apart from thus being markers and possibly causal determinants of metabolic syndrome–related disorders, FFAs might furthermore influence cardiovascular risk by interacting with endothelial function, inflammation, or other components of lipid metabolism. In nondiabetic subjects, FFAs are associated with familial cardiovascular risk. To further elucidate whether FFAs might indeed be useful for risk stratification in patients with stable coronary heart disease, we studied the association of serum FFA levels with prognosis in the Long-term Success of Cardiologic Rehabilitation Therapy (KAROLA) cohort, a well-defined cardiovascular high-risk population with 8 years of follow-up for nonfatal secondary cardiovascular disease (CVD) events as well as mortality. Appreciating that whereas most circulating FFAs are bound to albumin, the unbound FFA fraction (FFA u ) might be more closely related to pathology in acute cardiovascular conditions, we additionally explored the prognostic value of the FFA/albumin ratio, a good proxy of FFA u levels.
Methods
Our analyses were based on data from the KAROLA study, which has been described in detail elsewhere. In brief, patients aged 30 to 70 years and admitted from January 1999 to May 2000 to 1 of 2 participating rehabilitation clinics for in-hospital rehabilitation within 3 months (mean 6 weeks) after acute cardiovascular events (acute myocardial infarction, coronary syndromes, or coronary artery intervention) were eligible for participation in this prospective follow-up study. Inclusion was conditional on written informed consent. The study protocol was approved by the ethics boards of the physicians’ chambers of Hessen and Baden-Württemberg and of the University of Ulm and the University of Heidelberg.
Baseline information was obtained from standardized self-administered questionnaires at the beginning of the rehabilitation program and from hospital records. Active follow-up was conducted 1, 3, 4.5, 6, and 8 years after baseline, obtaining information on incident secondary CVD events (myocardial infarction or stroke) from treating general practitioners and, in the case of deceased participants, death certificates indicating the cause of death from public health authorities.
Blood samples were taken at discharge from rehabilitation using serum Monovettes (Sarstedt AG & Company, Nümbrecht, Germany), centrifuged according to the manufacturer’s instructions, cooled at −20°C for a maximum of 4 weeks, mailed to the study center on dry ice, and stored at −80°C until analysis. FFAs were measured using the NEFA FS* kit (DiaSys Diagnostic Systems, Holzheim, Germany), which quantifies FFAs through an enzymatic end point method with manufacturer-provided intra- and interassay coefficients of variation of about 1.1%. Details of other laboratory measurements and respective assays have been described elsewhere.
To maximize the comparability with the most relevant previous publication, FFA quartiles were used in most analyses. The associations of baseline FFAs with secondary CVD events (nonfatal myocardial infarction or stroke, or death from CVD [up to year 4.5 of follow-up: International Classification of Diseases, Ninth Revision, codes 390 to 459; years 6 and 8 of follow-up: International Classification of Diseases, Tenth Revision, codes I00 to I99, 1 case of code R57.0]) and all-cause mortality were first examined by Kaplan-Meier plotting. In subsequent Cox regression analysis estimating hazard ratios (HR), which can be considered a measure of relative risk, model 1 was adjusted for age and gender; model 2 was additionally adjusted for the most important established risk indicators to investigate whether FFAs, regardless of causal considerations, could contribute to risk stratification beyond these traditional predictors of prognosis in patients with stable coronary heart disease (body mass index, history of diabetes, hypertension, and myocardial infarction, smoking status, renal function, discharge prescription of angiotensin-converting enzyme inhibitors, extent of heart failure as assessed by N-terminal–pro-brain natriuretic peptide); model 3 was additionally adjusted for markers of metabolic dysregulation to investigate if any association in model 2 would be attenuated by the inclusion of these additional potentially mediating variables (blood glucose, lipid-lowering drugs, high-density lipoprotein and total cholesterol, and triglycerides); and model 4 was adjusted like model 3 but excluded covariates with p values >0.20 to reduce possible overfitting. Strongly skewed continuous variables (N-terminal–pro-brain natriuretic peptide, fasting glucose, triglycerides, high-sensitivity C-reactive protein, and interleukin-6) were log transformed.
The proportional-hazards assumption was assessed by testing interaction terms of the main exposure with log(survival time) while adjusting for age and gender. The nonlinearity of associations of continuous covariates with outcomes was assessed in model 3 by including quadratic terms.
The potential effect heterogeneity of FFAs according to the presence of diabetes was tested by introducing an interaction term between these 2 variables in model 4. The dose-response relations of the main exposures with outcomes were examined using restricted cubic splines, a regression method that allows nonlinear associations between a risk factor and an outcome. Sensitivity analyses included additional adjustment for the potentially mediating variable cholesteryl ester transfer protein, or the inflammatory marker interleukin-6, or correcting smoking status on the basis of self-report through serum cotinine measurements.
As a surrogate for FFA u , the FFA/albumin ratio was also examined in the main models. In particular, its associations with the outcomes were examined after categorization into quartiles and adjusted like model 4, and its dose-response relation was studied in spline regression models. More sophisticated procedures predicting FFA u from parametric approximations of complex FFA/albumin binding dynamics have been developed but were disregarded here in favor of the FFA/albumin ratio put forward by the same group.
Results
Baseline FFA measurements were available for 1,045 of 1,091 subjects (96%) with secondary CVD follow-up and for 1,139 of 1,204 subjects (95%) with mortality follow-up (1 subject with an outlying FFA concentration of 3.44 mmol/L was excluded). The FFA concentrations were right skewed, with a geometric mean of 0.43 mmol/L (95% confidence interval [CI] 0.42 to 0.44) and quartile cutoffs of 0.32, 0.43, and 0.60 mmol/L.
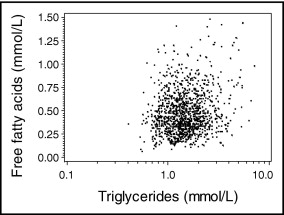
Table 1 lists the distributions of baseline characteristics and FFAs in the analysis population. Mean FFA level appeared higher in older subjects and in women and furthermore in the presence of components of the metabolic syndrome (obesity, diabetes, and hypertension). For various laboratory markers assessed in KAROLA, fairly pronounced correlations with FFAs emerged ( Table 2 ). Figure 1 shows a scatterplot of FFAs versus triglycerides.
| Characteristic | n | % | FFAs (mmol/L) | |
|---|---|---|---|---|
| Mean | 95% CI | |||
| Age (years) | ||||
| 30–39 | 27 | 2.4% | 0.38 | 0.30–0.49 |
| 40–49 | 149 | 13% | 0.40 | 0.36–0.43 |
| 50–59 | 334 | 29% | 0.41 | 0.39–0.44 |
| 60–70 | 629 | 55% | 0.45 | 0.43–0.47 |
| Women | 176 | 16% | 0.49 | 0.45–0.53 |
| Men | 963 | 85% | 0.42 | 0.41–0.43 |
| Acute coronary syndromes | 138 | 12% | 0.40 | 0.36–0.44 |
| Coronary intervention | 330 | 29% | 0.41 | 0.39–0.44 |
| Myocardial infarction | 671 | 59% | 0.44 | 0.43–0.46 |
| Body mass index (kg/m 2 ) | ||||
| ≤25 | 318 | 28% | 0.38 | 0.36–0.41 |
| >25–30 | 644 | 57% | 0.43 | 0.42–0.45 |
| >30 | 176 | 16% | 0.50 | 0.47–0.54 |
| Diabetes mellitus | 199 | 18% | 0.54 | 0.51–0.58 |
| Hypertension | 627 | 55% | 0.45 | 0.43–0.47 |
| Discharge prescriptions | ||||
| Angiotensin-converting enzyme inhibitors | 608 | 54% | 0.46 | 0.44–0.48 |
| Lipid-lowering drugs | 876 | 77% | 0.42 | 0.41–0.44 |
| Smoking | ||||
| Never | 350 | 31% | 0.45 | 0.43–0.48 |
| Formerly | 726 | 64% | 0.42 | 0.40–0.44 |
| Currently | 63 | 5.5% | 0.43 | 0.37–0.50 |
⁎ Reported are geometric means. Numbers do not always sum to 1,139, because of missing values for some covariates.
| Parameter | r | p |
|---|---|---|
| N-terminal–pro-brain natriuretic peptide | −0.033 | 0.27 |
| Fasting glucose | 0.195 | <0.0001 |
| High-density lipoprotein cholesterol | −0.017 | 0.57 |
| Total cholesterol | 0.016 | 0.59 |
| Triglycerides | 0.150 | <0.0001 |
| Cholesteryl ester transfer protein | −0.144 | <0.0001 |
| High-sensitivity C-reactive protein | 0.074 | 0.012 |
| Interleukin-6 | 0.093 | 0.0017 |
| Adiponectin | −0.097 | 0.0011 |
| Fetuin A | 0.071 | 0.017 |
| Adipocyte fatty acid–binding protein | 0.289 | <0.0001 |
| Retinol-binding protein-4 | −0.080 | 0.0072 |
| Lipoprotein-associated phospholipase A 2 | 0.056 | 0.058 |
| Secretory phospholipase A 2 | 0.075 | 0.011 |
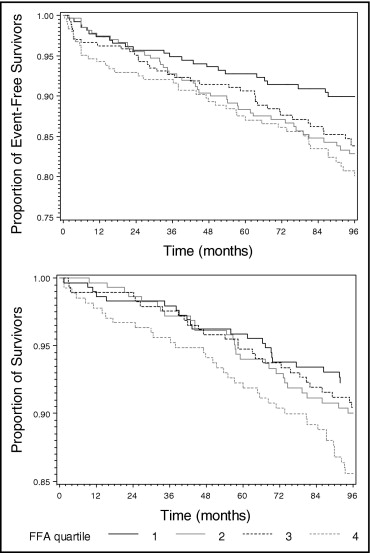
During a median of 8.1 years of follow-up, 153 nonfatal or fatal secondary CVD events occurred (51 nonfatal myocardial infarctions, 41 nonfatal strokes, and 61 cardiovascular deaths) and an additional 63 deaths due to noncardiovascular causes. Kaplan-Meier curves of the time to secondary CVD events or death from any cause suggested rather limited survival differences between subjects with different FFA concentrations ( Figure 2 ). In the Cox regression models adjusted only for age and gender, the risk for an adverse outcome appeared almost doubled in subjects in the fourth compared to the first quartile of FFAs ( Table 3 ). These associations, however, were strongly attenuated when adjusting for additional risk markers, and in the case of the all-cause mortality disappeared, suggesting that there might be only limited independent correlation of FFA with prognosis.
| Outcome | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Secondary CVD | ||||||||
| FFA ≤0.32 mmol/L | 1 | Reference | 1 | Reference | 1 | Reference | 1 | Reference |
| FFA >0.32–0.43 mmol/L | 1.65 | 1.01–2.70 | 1.50 | 0.90–2.49 | 1.39 | 0.83–2.32 | 1.40 | 0.84–2.33 |
| FFA >0.43–0.60 mmol/L | 1.51 | 0.91–2.49 | 1.36 | 0.81–2.30 | 1.33 | 0.79–2.25 | 1.31 | 0.78–2.21 |
| FFA >0.60 mmol/L | 1.94 | 1.18–3.17 | 1.43 | 0.84–2.44 | 1.33 | 0.78–2.29 | 1.34 | 0.79–2.27 |
| Death | ||||||||
| FFA ≤0.32 mmol/L | 1 | Reference | 1 | Reference | 1 | Reference | 1 | Reference |
| FFA >0.32–0.43 mmol/L | 1.34 | 0.77–2.31 | 1.17 | 0.66–2.07 | 1.09 | 0.61–1.94 | 1.05 | 0.60–1.85 |
| FFA >0.43–0.60 mmol/L | 1.25 | 0.72–2.17 | 1.04 | 0.58–1.86 | 0.96 | 0.53–1.72 | 0.94 | 0.53–1.67 |
| FFA >0.60 mmol/L | 1.87 | 1.11–3.17 | 1.22 | 0.69–2.16 | 1.04 | 0.58–1.88 | 1.09 | 0.62–1.91 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


