Pretreatment with antiP2Y 12 agents before angiography in acute coronary syndrome (ACS) is associated with a reduction in thrombotic events. However, recent evidences have questioned the benefits of upstream antiP2Y12, reporting a higher incidence of bleeding. We analyzed the prognostic impact of clopidogrel pretreatment in a large cohort of invasively managed patients with ACS. In hospital, safety and efficacy of clopidogrel pretreatment were retrospectively analyzed in patients included in the ARIAM-Andalucía Registry (Analysis of Delay in Acute Myocardial Infarction). Propensity score and inverse probability of treatment weighting analysis were performed to control treatment selection bias. Results were stratified by ACS type. Sensitivity analyses were used to explore stability of the overall treatment effect. Of 9,621 patients managed invasively, 69% received clopidogrel before coronary angiography. In the ST-elevation myocardial infarction group, pretreatment was associated with a significant reduction in reinfarction (odds ratio 0.53, 95% confidence interval [CI] 0.27 to 0.96; p = 0.027), stent thrombosis (odds ratio 0.15, 95% CI 0.06 to 0.38; p <0.0001), and mortality (odds ratio 0.67, 95% CI 0.48 to 0.94; p = 0.020), with an increase in minor bleeding but remained as a net clinical benefit strategy. Those benefits were not present in patients without ST elevation (non–ST elevation ACS). The weighting and propensity analysis confirmed the same results. An interaction between pretreatment duration and bleeding was observed. In conclusion, pretreatment with clopidogrel reduced the occurrence of death and thrombotic outcomes at the cost of minor bleeding. Those benefits exclusively affected ST-elevation myocardial infarction cases. The potential benefit of routine upstream pretreatment in patients with non–ST-elevation ACS should be reappraised at the present.
Dual antiplatelet therapy (DAPT) combining aspirin with an inhibitor of platelet P2Y 12 receptor is the cornerstone of antithrombotic treatment of acute coronary syndrome (ACS) and should be administered as soon as possible. But these recommendations do not reflect current clinical practice, in which time delays and percutaneous coronary intervention (PCI) techniques have been significantly improved, minimizing the potential benefit of pretreatment when PCI is performed early. In relation to primary PCI (pPCI) in ST-elevation myocardial infarction (STEMI), the data supporting pretreatment are weaker and based on post hoc analysis of randomized clinical trials or observational studies. However, based on the pathophysiology of STEMI that entails a higher thrombus burden and given the delay in the onset of action of antiP2Y 12 observed in the real world, pretreatment with antiP2Y 12 before pPCI is widely used, also supported by the low rate of urgent coronary artery bypass grafting (CABG) performed in STEMI. Recent results from the A Comparison of Prasugrel at the Time of Percutaneous Coronary Intervention or as Pretreatment at the Time of Diagnosis in Patients with Non-ST Elevation Myocardial Infarction (ACCOAST) study in which upstream therapy with prasugrel in non–ST-elevation ACS (NSTE ACS) was not associated with a reduction in the primary end point of efficacy but increased bleedings has revived controversy about the benefit of pretreatment before PCI. Pretreatment with ticagrelor has not been evaluated in NSTE ACS, and results in STEMI cases further confirm the lack of benefit of pretreatment with new antiP2Y 12 . We sought to evaluate the prevalence of pretreatment with clopidogrel before PCI and determine its efficacy and safety in patients with ACS scheduled for an invasive strategy.
Methods
We design a retrospective observational study of patients prospectively enrolled in the ARIAM-Andalucía Registry (Analysis of Delay in Acute Myocardial Infarction) between January 2002 and December 2012. The particularities of this registry have been previously described. In brief, demographic, laboratory, and angiographic variables and occurrence of clinical events during the hospital course were collected from patients with definitive diagnosis of ACS admitted to coronary care units of 49 centers through an online platform ( www.ariam-andalucia.org ). Patients with ACS in whom an invasive approach (coronary angiography) was performed during admission and who received clopidogrel constituted the study population. The principal investigators of each center gave its approval for data review and manuscript submission. The study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations for observational studies.
Clopidogrel pretreatment was defined as any dose of clopidogrel (300/600/or 75 mg in clopidogrel chronically treated patients) administered at the time of the first medical contact before coronary angiography or PCI. Patients who were treated with clopidogrel in the catheterization laboratory, either just before (<6 hours) or during PCI, constituted the no-pretreatment group. The duration of pretreatment was estimated as the time elapsed between first medical contact and coronary angiography. Total ischemic time was defined as the time from the onset of symptoms to PCI.
The end point adjudication was conducted and monitored by each local research group, being predefined in the data collection form. The primary efficacy end point was the occurrence of major cardiac and cerebrovascular events (MACCE) defined by the combination of cardiovascular death and nonfatal reinfarction or stroke/transient ischemic attack. Secondary efficacy outcomes were the occurrence of each of the components of MACCE, definitive stent thrombosis, according to the Academic Research Consortium definition, and cardiogenic shock. The primary safety end point was the occurrence of major, minor, or minimal bleeding according to the Thrombolysis In Myocardial Infarction criteria. The end point of net efficiency (net adverse clinical event [NACE]) was defined as the combination of the primary efficacy and the occurrence of any bleeding during hospitalization.
Data analysis was performed stratified by the type of ACS. Continuous variables are presented as mean ± SD or median (P25 and P75) when appropriate and compared using the unpaired student’s t test or its nonparametric equivalent (Mann-Whitney test). Categorical data are presented as frequencies and compared using the chi-square test or Fisher’s exact test. The association of clopidogrel pretreatment with the occurrence of MACCE, bleeding, and NACE was obtained by adjusted stepwise multivariate logistic regression models and expressed as adjusted odds ratio (OR) with their confidence interval (CI) at 95%. To control for treatment selection bias, 2 additional analyses were performed:
- (1)
Propensity analysis: Propensity score (PS) was defined as the probability of pretreatment with clopidogrel, according to a multivariate regression model in which 15 variables were included: age, gender, body mass index, type of ACS, diabetes, previous myocardial infarction, previous antiplatelet therapy, renal failure, previous oral anticoagulation, history of stroke/transient ischemic attack, Global Registry of Acute Coronary Events risk score, previous PCI, previous Chronic obstructive pulmonary disease (COPD), Killip class on admission, and transfer for Emergency Medical Systems. The matching was performed by the greedy method (1:1 without replacement). Goodness of fit was achieved if the standardized differences were ≤10%.
- (2)
Regression analysis adjusted by inverse probability of treatment weighting (IPTW) or regression analysis weighted by the inverse probability of treatment: The contribution of each subject or weighting in the pretreatment group was calculated as the inverse of PS [1/PS] and [1/1 − PS] for the control group. Such analysis ensures that the contribution of different input variables to construct the PS model does not differ between subjects in each group.
Several subgroups including inclusion time period (2002 to 2006 vs 2007 to 2012), dose of clopidogrel (600 vs ≤300 mg), time duration of pretreatment (<6 vs ≥6 h), and type of reperfusion in STEMI (pPCI vs fibrinolysis) were considered for sensitivity analysis, using the test for interaction. The discriminative ability and calibration power of the regression models were evaluated using the C statistic and the Hosmer-Lemeshow test, respectively. A 2-sided p value <0.05 was considered statistically significant. SPSS 19 (IBM Corporation, Somers, New York) and STATA 13 IC (STATA Corp., College Station, Texas) packages were used.
Results
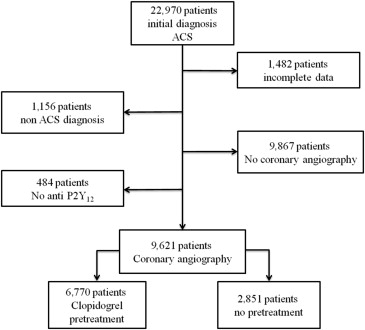
Of all patients included in the ARIAM-Andalucía Registry, 9,621 underwent coronary angiography and received clopidogrel, constituting the study population ( Figure 1 ). Of them, 6,770 (69%) received upstream pretreatment with clopidogrel and 2,851 (31%) did so during coronary angiography. Tables 1 and 2 list the baseline characteristics, pharmacologic treatment, and angiographic data according to the ACS type.
| Overall (n=9621) | STEMI | NSTE ACS | |||||
|---|---|---|---|---|---|---|---|
| Pretreatment (n=3973) | No pretreatment (n=2076) | p | Pretreatment (n=2797) | No Pretreatment (n=775) | p | ||
| Variables | |||||||
| Age (years) | 62±12 | 61±12 | 62±12 | 0.096 | 64±12 | 62±11 | 0.919 |
| Men | 7346 (76%) | 3149 (79%) | 1587 (76%) | 0.012 | 2050 (73%) | 560 (72%) | 0.732 |
| Current smokers | 3761 (39%) | 1751 (44%) | 885 (43%) | 0.121 | 901 (32%) | 224 (29%) | 0.073 |
| Diabetes mellitus | 3004 (31%) | 1104 (28%) | 616 (30%) | 0.224 | 989 (35%) | 295 (38%) | 0.171 |
| Dyslipidemia | 3970 (41%) | 1544 (39%) | 757 (36.5%) | 0.015 | 1344 (48%) | 315 (41%) | < 0.0001 |
| Hypertension | 5048 (52.5%) | 1875 (47%) | 1041 (50%) | 0.091 | 1684 (60%) | 448 (58%) | 0.199 |
| Obesity | 1495 (15.5%) | 738 (18.5%) | 118 (5.6%) | < 0.0001 | 576 (20%) | 63 (8%) | < 0.0001 |
| Prior myocardial infarction | 1389 (14.5%) | 409 (10%) | 306 (15%) | < 0.0001 | 490 (17,5%) | 184 (24%) | 0.001 |
| Chronic obstructive pulmonary disease | 493 (5%) | 187 (5%) | 40 (2%) | < 0.0001 | 226 (8%) | 40 (5%) | 0.003 |
| Chronic kidney disease | 244 (2.5%) | 72 (2%) | 14 (0.7%) | < 0.0001 | 138 (5%) | 20 (2.6%) | 0.003 |
| Stroke/ transient ischemic attack | 487 (5%) | 166 (4%) | 119 (6%) | 0.015 | 160 (6%) | 42 (5,4%) | 0.585 |
| Prior percutaneous coronary intervention | 1027 (11%) | 268 (6.7%) | 214 (10%) | < 0.0001 | 416 (15%) | 129 (17%) | 0.146 |
| Prior coronary by-pass | 213 (2.2%) | 51 (1.3%) | 40 (2%) | 0.03 | 91 (3,2%) | 31 (4%) | 0.264 |
| Prior antiplatelet tx | 2491 (26%) | 747 (18.8%) | 475 (23%) | < 0.0001 | 976 (35%) | 293 (38%) | 0.07 |
| Clinical presentation | |||||||
| STEMI | 6049 (63%) | 3973 (66%) | 2076 (34%) | < 0.0001 | — | — | — |
| NSTE ACS | 3572 (37%) | — | — | — | 2797 (78%) | 775 (22%) | < 0.0001 |
| Unstable angina | 1041 (11%) | 770 (27.5%) | 271 (35%) | <0.0001 | |||
| NSTEMI | 2531 (26%) | 2027 (72.5%) | 504 (65%) | <0.0001 | |||
| GRACE risk score | 133 [110, 159] | 139 [117, 164] | 136 [113, 166] | 0.986 | 123 [100, 150] | 127 [95, 162] | 0.090 |
| Killip class | 0.015 | 0.439 | |||||
| I-II | 8801 (91.5%) | 3645 (92%) | 1853 (89%) | 2590 (92.6%) | 713 (92%) | ||
| III-IV | 820 (8.5%) | 328 (8%) | 222 (11%) | 207 (7.4%) | 62 (8%) | ||
| Time period | < 0.0001 | < 0.0001 | |||||
| 2002/2006 | 2475 (26%) | 272 (7%) | 1563 (75%) | 98 (3.5%) | 542(70%) | ||
| 2007/2012 | 7146 (74%) | 3701 (93%) | 513 (25%) | 2699 (96.5%) | 233 (30%) | ||
| Variables | Overall (n=9621) | STEMI | NSTE ACS | ||||
|---|---|---|---|---|---|---|---|
| Pretreatment (n=3973) | No pretreatment (n=2076) | p | Pretreatment (n=2797) | No pretreatment (n=775) | p | ||
| Drugs ∗ | |||||||
| IIb-IIIa Glicoprotein inhibitors | 2680 (29%) | 1070 (27%) | 575 (27.3%) | 0.973 | 780 (28%) | 255 (33%) | 0.059 |
| Unfractionated heparin | 1040 (11%) | 508 (13%) | 391 (19%) | < 0.0001 | 77 (2.7%) | 64 (8.2%) | < 0.0001 |
| Low molecular weight heaprin | 7575 (78%) | 3075 (77.4%) | 1492 (72%) | 0.009 | 2383 (85%) | 625 (80%) | 0.219 |
| Fondaparinux | 612 (6%) | 258 (6,5%) | 33 (1.6%) | < 0.0001 | 305 (11%) | 16 (2%) | < 0.0001 |
| Bivalirudin | 64 (0.7%) | 39 (0.9%) | 21 (1%) | 0.990 | 4 (0,1%) | — | 0.279 |
| Thrombolysis | 2588 (27%) | 1736 (43.5%) | 852 (41%) | 0.048 | — | — | — |
| Clopidogrel (load dose) | < 0.0001 | 0.001 | |||||
| ≤300 mg | 8771 (91%) | 3377 (85%) | 1959 (94,4%) | 2713 (97%) | 722 (93%) | ||
| 600 mg | 850 (9%) | 596 (15%) | 117 (5.6%) | 84 (3%) | 53 (7%) | ||
| Clopidogrel-to-angiography (hours) † | 12 [1.8, 50] | 3 [1.2, 19] | — | — | 42 [21, 73] | — | — |
| Angiography/PCI | |||||||
| Symptons-to- angiography (hours) | 15 [4, 52] | 6 [3.3,22] | 6,3 [3, 33] | 0.175 | 47 [26, 80] | 52 [24, 135] | 0.193 |
| PCI ( with stent) | 8434 (88%) | 3705 (93%) | 1857 (89.4%) | < 0.0001 | 2271 (81%) | 601 (77.5%) | 0,024 |
| Bare metal stent | 3974 (41%) | 1667 (42%) | 1021 (49%) | < 0.0001 | 931 (33%) | 355 (45.8%) | < 0.0001 |
| Drug eluting stent | 4460 (46%) | 2038 (51%) | 836 (40%) | < 0.0001 | 1340 (48%) | 246 (32%) | < 0.0001 |
| Bare metal+Drug eluting stent | 436 (4.5%) | 167 (4.2%) | 120 (5.8%) | 0.004 | 121 (4.3%) | 28 (3.6%) | 0.417 |
| No. of stents | 1.7±1 | 1.7±1 | 1.6±1 | 0.001 | 1.8±2 | 1.6±1 | 0.004 |
| No. Vessel disease | |||||||
| 0 | 593 (6%) | 175 (4.4%) | 55 (2.6%) | 0.0006 | 304 (11%) | 59 (7.6%) | 0.072 |
| 1 | 5124 (53%) | 2423 (61%) | 1287 (62%) | 0.453 | 1119 (40%) | 295 (38%) | 0.340 |
| 2 | 2439 (25%) | 914 (23%) | 540 (26%) | 0.01 | 727 (26%) | 258 (33.4%) | < 0.0001 |
| 3 | 1465 (16%) | 461 (11.6%) | 194 (9.4%) | 0.007 | 647 (23%) | 163 (21%) | 0.226 |
| Left main | 442 (4.6%) | 172 (4.3%) | 39 (2%) | < 0.0001 | 202 (7.2%) | 29 (3.7%) | 0.004 |
| Primary PCI | 3193 (33%) | 2048 (51.5%) | 1145 (55%) | 0.008 | — | — | — |
| Rescue PCI | 1372 (14%) | 1190 (30%) | 182 (9%) | < 0.0001 | — | — | — |
| Angiography without PCI | 1071 (11%) | 242 (6%) | 186 (9%) | < 0.0001 | 492 (17.6%) | 151 (19.5%) | 0.205 |
| Coronary by-pass | 116 (1.2%) | 26 (0.8%) | 33 (1.6%) | < 0.0001 | 34 (1.2%) | 23 (3%) | 0.001 |
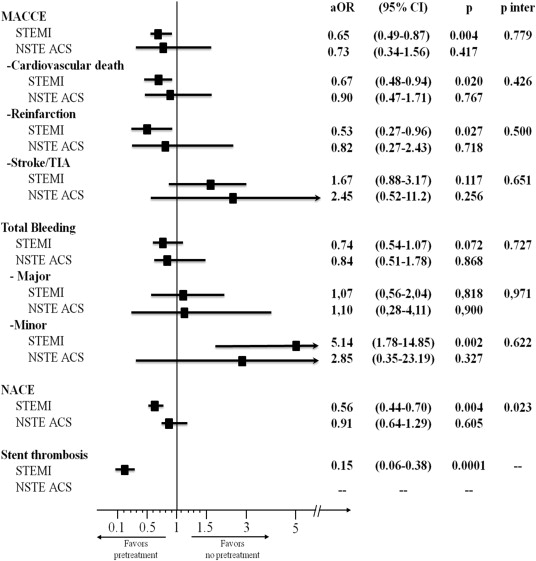
Clinical outcomes in the pooled group are listed in Table 3 . In the pretreated group, fewer MACCE was observed, with a significant reduction in the rate of reinfarction and stent thrombosis, with a numerical reduction in the mortality and a nominal increase in the rate of stroke, mainly driven by hemorrhagic stroke, 17 cases (0.25%) in pretreated versus 2 patients (0.07%) in the non–pretreated group; p = 0.019, with no difference in the rate of ischemic stroke (0.74% vs 0.6%, p = ns). The higher rate of hemorrhagic stroke was seen in the subgroup of patient with ST elevation pretreated with clopidogrel who received fibrinolysis (0.2% vs 0%, p = 0.013). The STEMI subgroup pretreated with clopidogrel showed the same reduction in ischemic events observed in the overall population, with a numerical reduction in mortality and a significant reduction in stent thrombosis.
| Outcome ∗ | Overall (n=9621) | STEMI (n=6049) | NSTE ACS (n=3572) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment (n=6770) | No pretreatment (n=2851) | p | Pretreatment (n=3973) | No pretreatment (n=2076) | p | Pretreatment (n=2797) | No pretreatment (n=775) | p | |
| MACCE | 318 (4.7%) | 166 (5.8%) | 0.021 | 229 (5.8%) | 143 (7%) | 0.084 | 89 (3.2%) | 23 (3%) | 0.762 |
| Death | 262 (3.9%) | 133 (4.6%) | 0.113 | 188 (4.7%) | 116 (5.6%) | 0.123 | 74 (2.6%) | 17 (2.2%) | 0.246 |
| Reinfarction | 32 (0.5%) | 25 (0.9%) | 0.031 | 22 (0.5%) | 20 (0.9%) | 0.040 | 5 (0.18%) | 10 (1.3%) | < 0.0001 |
| Stroke/TIA | 52 (0.8%) | 15 (0.5%) | 0.041 | 41 (1%) | 14 (0.7%) | 0.199 | 11 (0.4%) | 1 (0.13%) | 0.481 |
| Bleeding | 223 (3.3%) | 140 (4.9%) | < 0.0001 | 139 (3.5%) | 113 (5.4%) | 0.0004 | 84 (3%) | 27 (3.5%) | 0.235 |
| Major | 36 (0.5%) | 19 (0.7%) | 0.926 | 26 (0.6%) | 16 (0.8%) | 0.818 | 10 (0.36%) | 3 (0.38%) | 0.891 |
| Minor | 41 (0.6%) | 5 (0.2%) | 0.001 | 31 (0.8%) | 4 (0.2%) | < 0.0001 | 10 (0.36%) | 1 (0.13%) | 0.309 |
| Minimal | 145 (2%) | 75 (2.6%) | 0.964 | 85 (2%) | 62 (3%) | 0.580 | 60 (2%) | 13 (1.7%) | 0.404 |
| Fatal | 10 (0.15%) | 3 (0.1%) | 0.583 | 7 (0.17%) | 3 (0.14%) | 0.782 | 3 (0.11%) | — | 0.335 |
| NACE | 499 (7.4%) | 282 (10%) | < 0.0001 | 340 (8.6%) | 235 (11.3%) | 0.0006 | 159 (5.7%) | 47 (6%) | 0.688 |
| Stent thrombosis | 7 (0.24%) | 42 (0.62%) | 0.003 | 7 (0.17%) | 42 (2%) | < 0.0001 | — | — | — |
| Cardiogenic shock | 398 (6%) | 193 (6.8%) | 0.097 | 304 (7.7%) | 166 (8%) | 0.635 | 94 (3.4%) | 27 (3.5%) | 0.867 |
Total bleeds were lower in the pretreatment group, with a significant increase in minor bleeding but without significant increased in fatal bleeding ( Table 3 ). These trends were also observed in STEMI subgroup without significant differences in the NSTE ACS subgroup. The subgroup of patients with STEMI who received clopidogrel pretreatment and lytics experienced higher rates of minor bleeding (0.3% vs 0.1%, p = 0.026) with no differences in total major or fatal bleeding, although with higher rates of intracranial hemorrhages.
The NACE outcome was favorable in the group that received pretreatment but only in the subgroup with ST elevation, without evidence of net clinical benefit in the NSTE ACS group ( Table 3 ).
In the adjusted multivariate analysis ( Figure 2 ), pretreatment with clopidogrel was associated with a significant reduction in MACCE including reinfarction, mortality, and stent thrombosis but only in the STEMI group, at the expense of a significant increase in minor bleeding, which resulted in a favorable net clinical benefit (p of interaction = 0.023).