Chapter 11 Principles of Preoperative and Operative Surgery
Principles of, and Preparation for, Operative Surgery
Preoperative Evaluation
The aim of preoperative evaluation is not to screen broadly for undiagnosed disease, but to identify and quantify any comorbidity that may affect the operative outcome. This evaluation is driven by findings on the history and physical examination suggestive of organ system dysfunction or by epidemiologic data suggesting the benefit of evaluation based on age, gender, or patterns of disease progression. The goal is to uncover problem areas that may require further investigation or be amenable to preoperative optimization (Table 11-1).1 Routine preoperative testing is not cost-effective and, even in older adults, is less predictive of perioperative morbidity than the American Society of Anesthesiologists (ASA) status or American Heart Association (AHA)/American College of Cardiology (ACC) guidelines for surgical risk.
Table 11-1 Suggestions for Adult Preoperative Testing
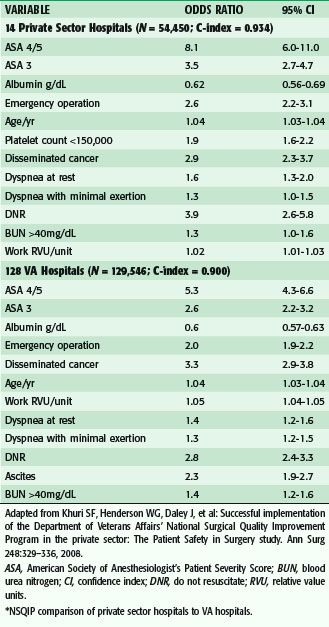
The preoperative evaluation is determined in light of the risk of the planned procedure (low, medium, or high), planned anesthetic technique, and postoperative disposition of the patient (outpatient or inpatient, ward bed, or intensive care). In addition, the preoperative evaluation is used to identify patient risk factors for postoperative morbidity and mortality. Along with being a generally accepted program for risk adjustment to monitor and improve surgical outcomes, The National Surgical Quality Improvement Program (NSQIP) has been used to develop predictive models for postoperative morbidity and mortality, and several factors have consistently been found to be independent predictors of postoperative events (Table 11-2), both in the Department of Veterans Affairs (VA) and in a more recent comparison between the VA and private sector hospitals.2 It is important to understand that the NSQIP has been validated as an excellent quality improvement tool by accounting for the influence of patient risk on outcomes from surgery and allowing hospitals to compare their outcomes to that of their peers. Although risk prediction models have been developed and are available to use in the VA, they have yet to be validated prospectively as they might apply to individual patients. The potential ability to predict an individual patient’s risk may have its biggest impact in allowing the surgeon to intervene with measures shown to decrease that risk.
Table 11-2 Top Patient Risk Factors Most Predictive of PostOperative Mortality*
| VARIABLE | ODDS RATIO | 95% CI |
|---|---|---|
| 14 Private Sector Hospitals (N = 54,450; C-index = 0.934) | ||
| ASA 4/5 | 8.1 | 6.0-11.0 |
| ASA 3 | 3.5 | 2.7-4.7 |
| Albumin g/dL | 0.62 | 0.56-0.69 |
| Emergency operation | 2.6 | 2.2-3.1 |
| Age/yr | 1.04 | 1.03-1.04 |
| Platelet count <150,000 | 1.9 | 1.6-2.2 |
| Disseminated cancer | 2.9 | 2.3-3.7 |
| Dyspnea at rest | 1.6 | 1.3-2.0 |
| Dyspnea with minimal exertion | 1.3 | 1.0-1.5 |
| DNR | 3.9 | 2.6-5.8 |
| BUN >40mg/dL | 1.3 | 1.0-1.6 |
| Work RVU/unit | 1.02 | 1.01-1.03 |
| 128 VA Hospitals (N = 129,546; C-index = 0.900) | ||
| ASA 4/5 | 5.3 | 4.3-6.6 |
| ASA 3 | 2.6 | 2.2-3.2 |
| Albumin g/dL | 0.6 | 0.57-0.63 |
| Emergency operation | 2.0 | 1.9-2.2 |
| Disseminated cancer | 3.3 | 2.9-3.8 |
| Age/yr | 1.04 | 1.03-1.04 |
| Work RVU/unit | 1.05 | 1.04-1.05 |
| Dyspnea at rest | 1.4 | 1.2-1.6 |
| Dyspnea with minimal exertion | 1.3 | 1.2-1.5 |
| DNR | 2.8 | 2.4-3.3 |
| Ascites | 2.3 | 1.9-2.7 |
| BUN >40mg/dL | 1.4 | 1.2-1.6 |
ASA, American Society of Anesthesiologist’s Patient Severity Score; BUN, blood urea nitrogen; CI, confidence index; DNR, do not resuscitate; RVU, relative value units.
* NSQIP comparison of private sector hospitals to VA hospitals.
Adapted from Khuri SF, Henderson WG, Daley J, et al: Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: The Patient Safety in Surgery study. Ann Surg 248:329–336, 2008.
The letter “E” is added to any of these for an emergency operation. Even though the system seems subjective, it continues to be a significant independent predictor of mortality.2 Although the ASA class should be determined for each patient, a more in-depth assessment of risk is indicated for procedures more involved than a skin biopsy.
Systems Approach to Preoperative Evaluation
Cardiovascular System
Assessment tools for stratification of the cardiovascular portion of anesthetic risk have been available for some time. The premiere example is Goldman’s criteria of cardiac risk for noncardiac surgery (Table 11-3).3 This strategy, designed by multivariate analysis, assigns points to easily reproducible characteristics. The points are then added to yield a total, which has been correlated with perioperative cardiac risk. One of the more important contributions of this work was the inclusion of functional capacity, clinical signs and symptoms, and operative risk assessment to estimate the patient’s overall risk and plan preoperative interventions. This concept has been further refined in the Revised Cardiac Risk Index, which uses six predictors of complications to estimate cardiac risk in noncardiac surgical patients, and is also shown in Table 11-3. In addition, several other investigators have proposed cardiac risk indices; however, many were found to be expensive and time-consuming.
Table 11-3 Cardiac Risk Indices
| CARDIAC RISK INDEX WITH VARIABLES | POINTS | COMMENTS |
|---|---|---|
| Goldman Cardiac Risk Index, 1977 | Cardiac complication rate | |
| 1. Third heart sound or jugular venous distention | 11 | 0-5 points = 1% |
| 2. Recent myocardial infarction | 10 | 6-12 points = 7% |
| 3. Nonsinus rhythm or premature atrial contraction on ECG | 7 | 13-25 points = 14% |
| 4. >5 premature ventricular contractions | 7 | >26 points = 78% |
| 5. Age >70 yr | 5 | |
| 6. Emergency operations | 4 | |
| 7. Poor general medical condition | 3 | |
| 8. Intrathoracic, intraperitoneal, or aortic surgery | 3 | |
| 9. Important valvular aortic stenosis | 3 | |
| Detsky Modified Multifactorial Index, 1986 | Cardiac complication rate | |
| 1. Class 4 angina | 20 | >15 = high risk |
| 2. Suspected critical aortic stenosis | 20 | |
| 3. Myocardial infarction within 6 mo | 10 | |
| 4. Alveolar pulmonary edema within 1 wk | 10 | |
| 5. Unstable angina within 3 mo | 10 | |
| 6. Class 3 angina | 10 | |
| 7. Emergency surgery | 10 | |
| 8. Myocardial infarction >6 mo ago | 5 | |
| 9. Alveolar pulmonary edema resolved >1 wk ago | 5 | |
| 10. Rhythm other than sinus or PACs on ECG | 5 | |
| 11. >5 PVCs any time before surgery | 5 | |
| 12. Poor general medical status | 5 | |
| 13. Age >70 yr | 5 | |
| Eagle’s Criteria for Cardiac Risk Assessment, 1989 | ||
| 1. Age >70 yr | 1 | <1, no testing |
| 2. Diabetes | 1 | 1-2, send for noninvasive test |
| 3. Angina | 1 | ≥3, send for angiography |
| 4. Q waves on ECG | 1 | |
| 5. Ventricular arrhythmias | 1 | |
| Revised Cardiac Risk Index | ||
| 1. Ischemic heart disease | 1 | Each increment in points increases risk for postoperative myocardial morbidity |
| 2. Congestive heart failure | 1 | |
| 3. Cerebral vascular disease | 1 | |
| 4. High-risk surgery | 1 | |
| 5. Preoperative insulin treatment of diabetes | 1 | |
| 6. Preoperative creatinine level >2 mg/dL | 1 |
PAC, Premature atrial contraction; PVC, premature ventricular contraction.
Adapted from Akhtar S, Silverman DG: Assessment and management of patients with ischemic heart disease. Crit Care Med 32(Suppl):S126–S136, 2004.
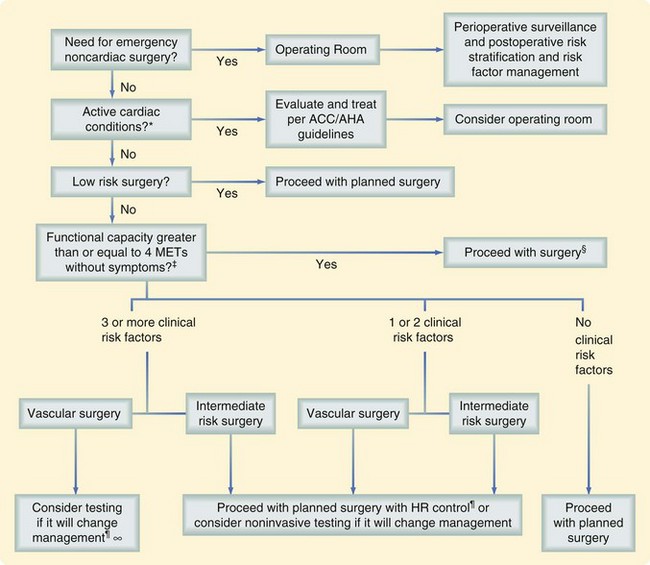
In an attempt to assess and optimize the cardiac status of patients undergoing noncardiac surgery, a joint committee of the ACC and AHA has developed an easily used tool (Fig. 11-1).4 This methodology takes into account previous coronary revascularization and evaluation and clinical risk assessment, divided into major, intermediate, and minor clinical predictors. The next factor taken into account is the patient’s functional capacity, which is estimated by obtaining a history of the patient’s daily activities. The earlier mentioned variables and type of surgery are then used to determine whether the pretest probability can be altered by noninvasive testing.
The standard exercise stress test, with or without thallium for perfusion imaging, can be limited by the functional capacity of the patient. Patients not able to exercise to an acceptable stress level may require pharmacologic stress testing with dipyridamole; thereafter, perfusion defects can be assessed via thallium or a dobutamine-induced stress, followed by functional evaluation with echocardiography. Angiography can then be used to define the anatomic abnormalities contributing to the ischemia exactly. Although no large prospective randomized trial has been conducted to determine whether following these guidelines result in improved outcomes for patients, several studies have suggested that it is useful to do so.3
Once these data have been obtained, the surgeon and consultants need to weigh the benefits of surgery against the risk and determine whether any perioperative intervention will reduce the probability of a cardiac event. The intervention usually centers on coronary revascularization via coronary artery bypass or percutaneous transluminal coronary angioplasty but may include modification of the choice of anesthetic or use of invasive intraoperative monitoring. Patients who have undergone a percutaneous coronary intervention with stenting need to have elective noncardiac procedures delayed for 4 to 6 weeks, although the delay may be shortened depending on the type of stent used (drug-eluting versus non–drug-eluting stent).3
The optimal timing of a surgical procedure after myocardial infarction (MI) is dependent on the duration of time since the event and assessment of the patient’s risk for ischemia, by clinical symptoms or noninvasive study. Any patient can be evaluated as a surgical candidate after an acute MI (within 7 days of evaluation) or a recent MI (within 7 to 30 days of evaluation). The infarction event is considered a major clinical predictor in the context of ongoing risk for ischemia. General recommendations are to wait 4 to 6 weeks after MI to perform elective surgery.3
Interventions with medical therapy have also been recommended, in particular beta blocker therapy. The underpinning of this type of therapy centered on decreasing the adrenergic surge associated with surgery and halting platelet activation and microvascular thrombosis. A 1996 study showed that perioperative risk for cardiovascular morbidity and mortality was decreased by 67% and 55%, respectively in ACC/AHA-defined medium- to high-risk patients receiving beta blockers in the perioperative period versus those receiving placebo. Although the benefit was most noticeable in the 6 months after surgery, event-free survival was significantly better in the group that received beta blockers up to 2 years after surgery.5 In 2007, the results of another large randomized trial (PeriOperative ISchemic Evaluation—POISE) showed the potential harm of perioperative beta blocker therapy.6 The POISE trial enrolled over 8000 patients undergoing noncardiac surgery. Although the results confirmed the reduction in perioperative cardiac events such as myocardial infarction, cardiovascular death, and cardiac arrest, this benefit was offset by an increased rate of stroke and total mortality with perioperative beta blocker therapy. Unlike the prior study, this study started high-dose, extended-release metoprolol on the day of surgery. The results were important enough to stimulate the ACC/AHA to modify their recommendations (Table 11-4).7 The current recommendations are to continue beta blockers for those who are on them preoperatively, consider them for high-risk patients (more than one risk factor), titrating to heart rate and blood pressure, and not to give them to low-risk patients.
Table 11-4 American Heart Association/American College of Cardiology Perioperative Focused Update Recommendations
| CURRENT RECOMMENDATION | CHANGE FROM PREVIOUS RECOMMENDATION |
|---|---|
| Class I* | |
| Beta blockers should be continued in patients undergoing surgery who are receiving beta blockers for treatment of conditions with ACCF/AHA class I guideline indications for the drugs. | Revised wording; recommendation of giving beta blockers to high cardiac risk vascular patients with findings of ischemia on preoperative testing moved down in class of recommendation (see class IIa) |
| Class IIa | |
| 1. Beta blockers titrated to heart rate and blood pressure are probably recommended for patients undergoing vascular surgery who are at high cardiac risk because of coronary artery disease or the finding of cardiac ischemia on preoperative testing. | Modified or combined recommendation, moved down in classification |
| 2. Beta blockers titrated to heart rate and blood pressure are reasonable for patients in whom preoperative assessment for vascular surgery identifies high cardiac risk, as defined by the presence of more than one clinical risk factor. | Modified recommendation (added “titrated to heart rate and blood pressure” and changed “are probably recommended” to “are reasonable”) |
| 3. Beta blockers titrated to heart rate and blood pressure are reasonable for patients in whom preoperative assessment identifies coronary artery disease or high cardiac risk, as defined by the presence of more than one clinical risk factor, who are undergoing intermediate-risk surgery. | Revised wording |
| Class IIb | |
| 1. The usefulness of beta blockers is uncertain for patients who are undergoing intermediate-risk procedures or vascular surgery in whom preoperative assessment identifies a single clinical risk factor in the absence of coronary artery disease. | Revised wording |
| 2. The usefulness of beta blockers is uncertain in patient undergoing vascular surgery with no clinical risk factors who are not currently taking beta blockers. | No change from 2007 recommendations |
| Class III | |
| 1. Beta blockers should not be given to patients undergoing surgery who have absolute contraindications to beta blockade. | No changes from 2007 recommendations |
| 2. Routing administration of high-dose beta blockers in the absence of dose titration is not useful and may be harmful to patients not currently taking beta blockers who are undergoing noncardiac surgery. | New recommendation |
* Class of recommendation is based on the size of the treatment effect combined with an estimate of certainty (precision) of the treatment effect. Clinical risk factors include history of ischemic heart disease, history of compensated or prior heart failure, history of cerebrovascular disease, diabetes mellitus, and renal insufficiency (defined in the revised cardiac risk index as a preoperative serum creatinine level >2 mg/dL).
Adapted from Fleischmann KE, Beckman JA, Buller CE, et al: 2009 ACCF/AHA focused update on perioperative beta blockade. J Am Coll Cardiol 54:2102–2128, 2009.
An easy and inexpensive method to determine cardiopulmonary functional status for noncardiac surgery is the patient’s ability or inability to climb two flights of stairs. Two flights of stairs are needed because it demands more than 4 metabolic equivalents (METs). In a review of all studies of stair climbing as preoperative assessment, prospective studies have shown it to be a good predictor of mortality associated with thoracic surgery.8 In major noncardiac surgery, an inability to climb two flights of stairs is an independent predictor of perioperative morbidity, but not mortality.
Pulmonary System
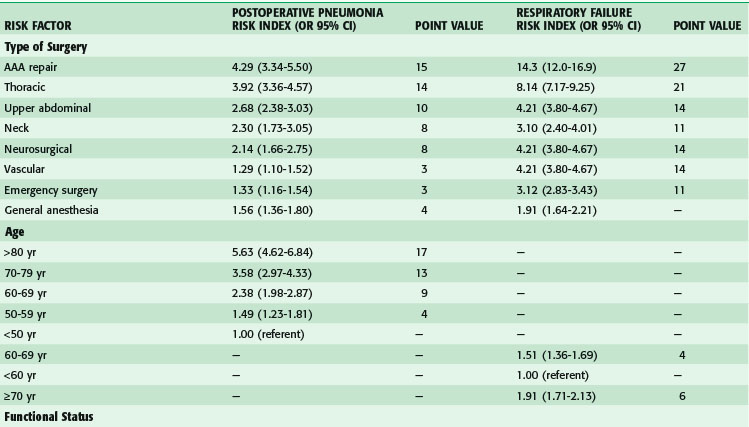
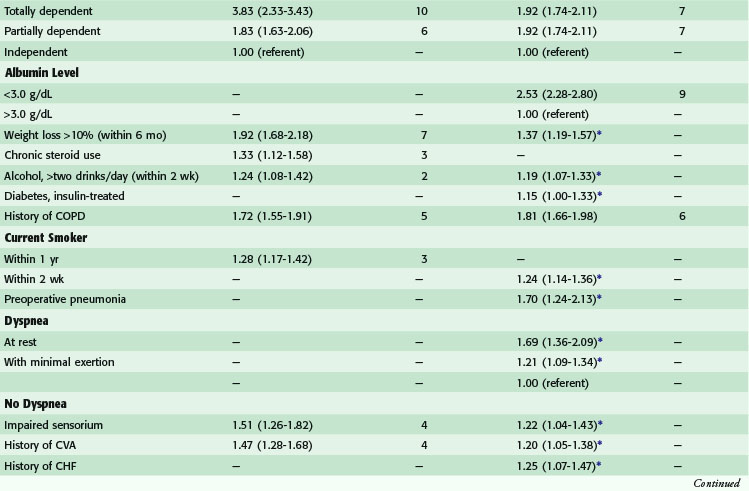
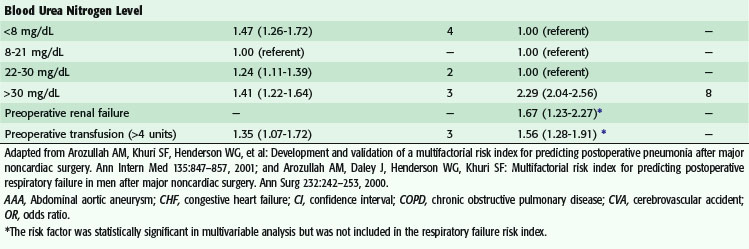
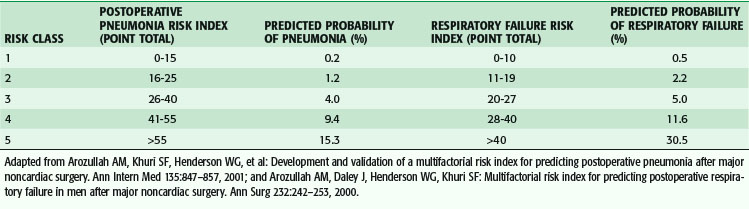
Postoperative pulmonary complications carry with them great cost—estimated to be over $50,000—and increased short- and long-term mortality.9,10 Risk factors for the development of postoperative pulmonary complications have been identified in a large population of VA patients (Tables 11-5 and 11-6), and recently confirmed in a mixed population. Whereas the VA population was fairly homogeneous, the Patient Safety in Surgery study11 included a more diverse group. Even with the diversity, the rates of pulmonary complications were not much different and the risk factors were very similar. Preoperative pulmonary assessment determines not only factors that confer increased risk but also potential targets to reduce the risk for pulmonary complications. General factors that increase risk for postoperative pulmonary complications include increasing age, lower albumin level, dependent functional status, weight loss, and possibly obesity. Concurrent comorbid conditions such as impaired sensorium, previous stroke, congestive heart failure, acute renal failure, chronic steroid use, and blood transfusion are also associated with increased risk for postoperative pulmonary complications. Specific pulmonary risk factors include chronic obstructive pulmonary disease, smoking, preoperative sputum production, pneumonia, dyspnea, and obstructive sleep apnea.
Renal System
Patients with chronic end-stage renal disease undergo dialysis before surgery to optimize their volume status and control the potassium level. Intraoperative hyperkalemia can result from surgical manipulation of tissue or transfusion of blood. Such patients are often dialyzed on the day after surgery as well. In the acute setting, patients who have a stable volume status can undergo surgery without preoperative dialysis, provided that no other indication exists for emergency dialysis.12 Prevention of secondary renal insults in the perioperative period include the avoidance of nephrotoxic agents and maintenance of adequate intravascular volume throughout this period. In the postoperative period, the pharmacokinetics of many drugs may be unpredictable, and adjustments in dosage need to be made according to pharmacy recommendation. Notably, narcotics used for postoperative pain control may have prolonged effects despite hepatic clearance, and nonsteroidal agents are avoided in patients with renal insufficiency.
Hepatobiliary System
Hepatic dysfunction may reflect the common pathway of a number of insults to the liver, including viral-, drug-, and toxin-mediated disease. A patient with liver dysfunction requires careful assessment of the degree of functional impairment, as well as a coordinated effort to avoid additional insult in the perioperative period (Fig. 11-2).13
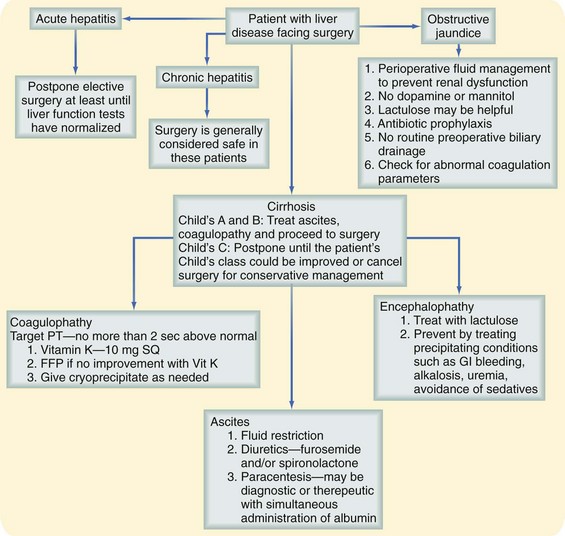
FIGURE 11-2 Approach to a patient with liver disease. FFP, fresh-frozen plasma; GI, Gastrointestinal; SQ, subcutaneous.
(Adapted from Rizvon MK, Chou CL: Surgery in the patient with liver disease. Med Clin North Am 87:211–227, 2003.)
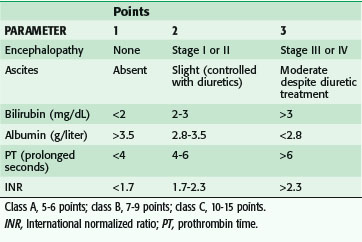
In the event of an emergency situation requiring surgery, such an investigation may not be possible. A patient with acute hepatitis and elevated transaminase levels is managed nonoperatively, when feasible, until several weeks beyond normalization of laboratory values. Urgent or emergency procedures in these patients are associated with increased morbidity and mortality. A patient with evidence of chronic hepatitis may often safely undergo surgery. A patient with cirrhosis may be assessed with the Child-Pugh classification, which stratifies operative risk according to a score based on abnormal albumin and bilirubin levels, prolongation of the prothrombin time (PT), and degree of ascites and encephalopathy (Table 11-7). This scoring system was initially used to predict mortality in cirrhotic patients undergoing portacaval shunt procedures, although it has been shown to correlate with mortality in cirrhotic patients undergoing a wider spectrum of procedures as well. Data generated more than 25 years ago showed that patients with Child class A, B, and C cirrhosis had mortality rates of 10%, 31%, and 76%, respectively, during abdominal operations; these figures have been validated more recently.8 Although the figures may not represent current risk for all types of abdominal operations, little doubt exists that the presence of cirrhosis confers additional risk for abdominal surgery, proportional to the severity of disease. Other factors that affect outcomes in these patients are the emergency nature of a procedure, prolongation of the PT more than 3 seconds above normal and refractory to correction with vitamin K, and presence of infection.
Several reports have shown decreased rates of complication with laparoscopic procedures performed in cirrhotic patients. Among the best-described procedures is laparoscopic cholecystectomy performed in patients with Child class A through C. When compared with open cholecystectomy, lower morbidity in terms of blood loss and wound infection has been observed.13a
Endocrine System
Perioperative Diabetic Management
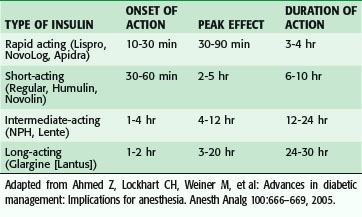
Insulin is available in several types and is typically classified by its length of action (Table 11-8). Rapid- and short-acting insulin preparations are usually withheld when the patient stops oral intake and are used for acute management of hyperglycemia during the NPO period. Intermediate- and long-acting insulin preparations are administered at two thirds the normal evening dose the night before surgery and half the normal morning dose the day of surgery, with frequent bedside glucose determinations and treatment with short-acting insulin, as needed. An infusion of 5% dextrose is initiated the morning of surgery.
Insulin pumps are used by some patients as their method of glucose management. These pumps use short-acting insulin (Velosulin) and have a variable delivery rate that can be programmed to simulate endogenous insulin production more closely. On the day of surgery, the patient continues with the basal insulin infusion. The pump is then used to correct the glucose level as it is measured. Patients generally have a correction or sensitivity factor that will decrease their glucose by 50 mg/dL. It is important to know this factor before the planned surgical procedure so that glucose can be managed in the operating room.14
Patients who take oral hypoglycemic agents (sulfonylureas, such as chlorpropamide and glyburide) typically withhold their normal dose the day of surgery. Patients can resume their oral agent once diet is resumed. An exception is metformin. If the patient has altered renal function, this agent needs to be discontinued until renal function normalizes or stabilizes to avoid potential lactic acidosis.15 Coverage for hyperglycemia is with a short-acting insulin preparation based on blood glucose monitoring.
Management of Other Endocrinopathies
A patient with a history of steroid use may require supplementation for a presumed abnormal adrenal response to perioperative stress (Box 11-1). Patients who have taken more than 5 mg of prednisone (or equivalent)/day for more than 3 weeks within the past year are considered at risk when undergoing major surgery. Lower doses of steroid or minor procedures are not generally associated with adrenal suppression.
BOX 11-1 Adapted from Schiff RL, Welsh GA: Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin North Am 87:175–192, 2003; and Kohl, BA, Schwartz S: Surgery in the patient with endocrine dysfunction. Med Clin North Am 93:1031–1047, 2009.
Perioperative Supplemental Glucocorticoid Regimens
No HPAA Suppression
Less than 5 mg of prednisone or equivalent/day for any duration
Alternate-day single morning dose of short-acting glucocorticoid of any dose or duration
Any dose of glucocorticoid for less than 3 wk
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree