Chapter 10 Primary Arterial Infections and Antibiotic Prophylaxis
Primary Arterial Infections
Historical Perspective
In the nineteenth century, Rokitansky1 and Koch2 recognized an association between arterial infection and aneurysm formation. In 1885, Osler3 presented the first comprehensive description of this relationship. In addressing the Royal College of Physicians, he described a 30-year-old man who had died from fever, chills, and pneumonia. At autopsy, the patient was found to have endocarditis involving the aortic valve, as well as multiple aneurysms of the thoracic aorta. Based on carefully described pathologic findings, Osler proposed a causal relationship between infection of the aortic wall and subsequent aneurysm formation. Because of a similarity between the beaded appearance of these aneurysms and fungal vegetations, he introduced the term mycotic aneurysm and thus the concept of primary arterial infection.
Pathogenesis
Oslerian Mycotic Aneurysms: Embolization of Infected Cardiac Vegetations
Considerable confusion has arisen because the term mycotic aneurysm has been expanded and applied to various types of infected aneurysms. Crane4 attempted to classify mycotic aneurysms as primary and secondary types. He introduced the term primary mycotic aneurysm to refer to infected aortic aneurysms not associated with endocarditis or an infectious focus; secondary types were those that formed as a result of preceding endocarditis. Ponfick5 and Eppinger6 were among the first to characterize the anatomic features of these aneurysms pathologically. Ponfick5 proposed that the initial insult to the arterial wall was a mechanical injury inflicted by the embolization of septic material. Eppinger in 1887 provided further support for the theory of septic emboli by culturing the same strain of bacteria from both vegetative lesions and the wall of an aneurysm in a patient with endocarditis. He applied the term embolomycotic to describe the combination of infectious and embolic components that led to the formation of mycotic aneurysms.6
Microbial Arteritis with Aneurysm Formation: Hematogenous Seeding
In 1906, the German pathologist Weisel7 described distinctive pathologic changes in arterial walls that occurred during the course of an infectious disease, but were not related to cardiac valve vegetation emboli.7 Lewis and Schrager8 and Cathcart9 presented case reports of infected peripheral aneurysms that developed in normal arteries of patients with osteomyelitis and typhoid fever, respectively. Despite these reports, nearly 30 years passed before consideration was given to the mechanism by which bacteremia led to arterial infection. Crane4 described an infected aneurysm in a patient with hypoplasia of the aorta, but no associated bacterial endocarditis or other identifiable source of infection.4 He proposed that the combination of the “force of the blood stream” and abnormal development of the aorta allowed bacteria to invade that portion of the aorta. This resulted in an arterial infection, disruption of the aortic wall, and an infected pseudoaneurysm. Revell extended the concept of aortic bacterial seeding one step further and proposed that the route of infection was through the aortic vasa vasorum.10 Hawkins and Yeager,11 acknowledging the resistance of arterial intima to infection, suggested that an intimal defect such as that produced by arteriosclerosis allowed bacterial localization and infection.
Arteritis from Contiguous Spread
Arterial infections can also develop through the spread of infection from a contiguous focus. Contiguous infections that have been recognized as potential sources of bacteria include lesions such as osteomyelitis, infected lymph nodes, tuberculous lymph nodes, and abscesses from narcotic injection.12 Bacteria, and less commonly mycobacteria or fungi, invade the artery either by direct extension or via lymphatics. They subsequently produce a necrotizing invasive infection of the arterial wall, with eventual destruction of the wall.
Other Forms of Arterial Infection
Syphilitic aneurysms are a rarely encountered complication of advanced syphilis. These lesions occur in approximately 10% of patients with the tertiary form of the disease.13 These aneurysms commonly arise in the ascending aorta, frequently involve the aortic valve, and are secondary to treponemal invasion of the vasa vasorum. The reasons why Treponema species prefers this portion of the aorta remain unclear. After spirochete penetration, an infiltrate develops within the vessel wall consisting of plasma cells, epidermal cells, and giant cells. This infiltrate results in destruction of the elastic and muscular components of the tunica media, replacement of the normal wall with fibrous tissue, and dilatation and subsequent formation of saccular aneurysms.
HIV-related vasculitis can result in aneurysmal degeneration of the arterial wall. Radiologic appearance may be identical to that which results from a bacterial arterial infection. The distinction is noted in the pathologic evaluation of the affected arterial wall, which demonstrates acute and chronic adventitial inflammatory changes. Secondary bacterial infections may be present, and cultures may be positive in a minority of these patients. There appears to be a predilection toward carotid and femoral arteries; however, the aorta may also be involved.14–16
Spontaneous or “primary” aortoenteric fistulas (AEFs) arise as a consequence of progressive aneurysmal enlargement, with gradual erosion into an adjacent segment of the gastrointestinal tract. The erosion is thought to be facilitated by the indurated, atherosclerotic artery pressing against a tethered portion of bowel. The most common location for this erosion is the third portion of the duodenum. In their 1951 review of a series of 16,633 autopsies, Hirst and Affeldt17 reported the incidence of this type of fistula to be 0.05%. Because the majority of patients with aortic aneurysms now undergo elective operation before they can progress to develop a primary aortoenteric fistula, the incidence of these lesions is thought to be considerably lower today. Patients with spontaneous AEFs may have an initial or “herald” bleed, which represents the initial hemorrhage of blood into the duodenum. In this presentation, the initial hemorrhage may abate then later resume in a more prolonged and dramatic manner. Presumably, clot within the AEF is responsible for the intermittent nature of the bleeding episodes. This condition is considerably different from that associated with aortic graft infection, or secondary AEF. Secondary AEF is more common, more dangerous, and more difficult to manage.
Graft excision and remote reconstruction are the standard management of secondary AEF. In contrast, significant evidence exists that primary AEF can be managed by closure of the duodenal rent, debridement of the aorta, and in situ reconstruction with an arterial prosthesis. The prerequisites of this approach are the absence of purulence at the fistula site, a small defect in the duodenum, and a relatively healthy patient. It should be noted that the management of primary aortic infections (mycotic aneurysms) is similar to that of primary AEF, in that the absence of gross infection along with adequate debridement may allow in situ graft reconstruction of the aorta.18
Causative Organisms
The organism most commonly associated with microbial aortitis is Salmonella. This is followed, in order of frequency, by Streptococcus species, Bacteroides species, Arizona hinshawii, Escherichia coli, and Staphylococcus aureus.18 Studies that focus on subpopulations such as intravenous (IV) drug abusers or those with femoral mycotic aneurysms tend to identify a predominance of gram-positive bacteria such as staphylococci and streptococci, along with gram-negative organisms such as E. coli and Pseudomonas species.
The bacteriology of primary arterial infections has undergone considerable transformation since its original description in the mid 1800s (Figure 10-1). Brown and colleagues19 suggested that the reason for this change is antibiotic selective pressure leading to bacterial adaptation. Also, there has been a change in the relative incidence of pathogenic mechanisms with the more common use of invasive diagnostic modalities, as well as the increased illicit use of IV drugs. The majority of arterial infections during the preantibiotic era were oslerian mycotic aneurysms; that is, they were related to bacterial endocarditis. The bacteriology of arterial infections during this period, therefore, was similar to that of endocarditis. Stengal and Wolferth20 in the 1920s and Revell10 in the 1940s reported that the predominant organisms were nonhemolytic streptococci, staphylococci, and pneumococci. Magilligan and Quinn,21 in a 1986 review, subdivided 91 patients with bacterial endocarditis into two groups: those known to be IV drug abusers (36 patients) and those who were not (55 patients). Of the first group, the most common organisms were S. aureus (36%), Pseudomonas species (16%), polymicrobial organisms (15%), Streptococcus faecalis (13%), and Streptococcus viridans (11%). Organisms in the second group (non-IV drug abusers) were S. viridans (22%), S. aureus (20%), S. faecalis (14%), and Staphylococcus epidermidis (11%). The declining incidence of rheumatic fever and the adoption of early, appropriate antibiotic treatment have resulted in a significant decrease in bacterial endocarditis. This in turn has resulted in a decline in the incidence of oslerian mycotic aneurysms in recent decades.
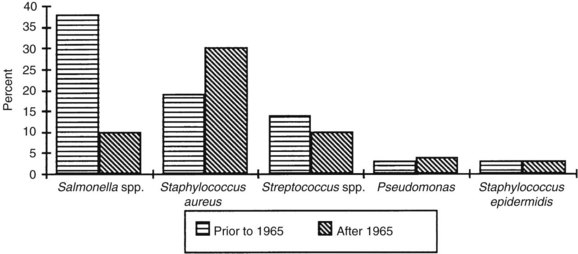
FIGURE 10-1 Organisms cultured from mycotic aneurysms.
(From Brown SL, Busuttil RW, Baker JD, et al: Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1:541, 1984.)
Concurrent with the declining incidence of oslerian mycotic aneurysms has been an increase in various other types of primary arterial infections. Principal among these are microbial arteritis and infected aneurysms. This may be due, in part, to the increasing age of the population and the simultaneous increase in the prevalence of atherosclerosis. The bacteriology of these arterial infections is different from that of oslerian mycotic aneurysms. The microorganisms most commonly associated with microbial arteritis are Salmonella species, Staphylococcus species, and E. coli. Salmonella species, in particular, have a striking propensity for invading diseased (atherosclerotic) aortas. In selected series, the involvement of Salmonella species has been reported to be as high as 50%. The most virulent species, Salmonella choleraesuis and Salmonella typhimurium, account for more than 60% of the reported cases of Salmonella arteritis.22 Less commonly reported organisms associated with microbial arteritis include fungi and anaerobic organisms. Among the latter, Bacteroides fragilis has been reported in association with supraceliac aortic aneurysms.
The bacteriology of infected aneurysms is similar to that of both mycotic aneurysms and microbial arteritis. Despite this, some variation exists among reported series. Although Bennett and Cherry23 reported a 66% incidence of Salmonella infections, Jarrett and associates24 described a predominance of gram-positive cocci (59%), with S. aureus representing 41%. In two prospective studies of patients undergoing aneurysmectomy, cultures obtained from both the aneurysm wall and the bowel bag revealed a predominance of gram-positive organisms.24,25 Both of these series are thought to represent cases of bacterial colonization. Despite the relative infrequency of gram-negative organisms observed in Jarrett’s series, the distinction between gram-negative and gram-positive cultures proved clinically important. Patients with gram-negative bacteria demonstrated a greater likelihood of aortic rupture than did those with gram-positive organisms. Specifically, the rupture rate associated with gram-negative bacterial isolates was 84%, whereas that associated with gram-positive bacterial cultures was 10%.
According to Brown and associates,19 the most common infected aneurysms since 1965 are those that occur as a result of mechanical arterial injury with contamination of the vessel wall. The organism most frequently implicated in this type of arterial infection is S. aureus, which Brown’s group cultured in as many as 30% of cases. Reddy and associates,26 in a series of infected femoral false aneurysms, reported a 65% incidence of S. aureus and a 33% rate of polymicrobial infection.26 Although arterial infections secondary to contiguous spread are most commonly bacterial, mycobacterial and fungal infections may also occur in these lesions. As with microbial arteritis, Salmonella organisms are the predominant pathogen, and Staphylococcus organisms are second in frequency (Figure 10-2).
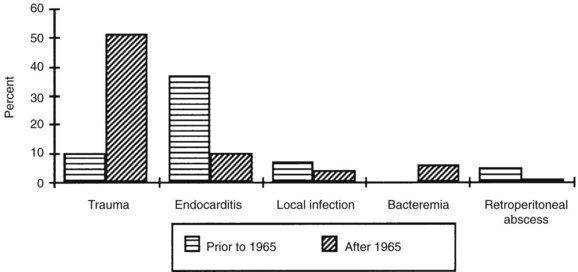
FIGURE 10-2 Causes of mycotic aneursyms.
(From Brown SL, Busuttil RW, Baker JD, et al: Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1:541, 1984.)
The increased number of immunosuppressed patients in certain settings has resulted in an increase of reports indicating opportunistic infections. Patients with cancer, prolonged steroid use, transplantation and other immunosuppressive conditions may present with infections. In these populations, infections from opportunistic agents are more prominent. Specifically noted are infections from Campylobacter species, Listeria species, and Mycobacterium tuberculosis.27
Anatomic Distribution
The anatomic distribution of primary arterial infections varies somewhat, depending on the pathologic type. True oslerian mycotic aneurysms most often involve the larger muscular and elastic arteries. In retrospective reviews, both Lewis and Schrager8 and Brown and colleagues19 found the most common sites of infection to be the abdominal aorta and the femoral and superior mesenteric arteries (Figure 10-3). The predisposition for aortic involvement is thought to be related to the higher incidence of underlying atherosclerotic aneurysms in this location compared with other anatomic sites.
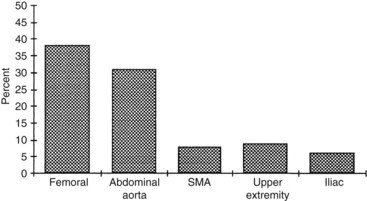
FIGURE 10-3 Distribution of mycotic aneurysms. SMA, Superior mesenteric artery.
(From Brown SL, Busuttil RW, Baker JD, et al: Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1:541, 1984.)
Diagnostic Testing
Blood Cultures
The demonstration of bacterial organisms in association with an arterial lesion is central to the diagnosis of an arterial infection. The bacteria may be detected by either blood cultures or cultures of the arterial wall itself. Blood cultures, by virtue of their availability, are frequently one of the first tests done in patients suspected of having a significant infection. If the patient is floridly bacteremic, the blood culture may detect the circulating bacteria. However, several problems limit the usefulness of blood cultures. The incidence of negative blood cultures testifies to the fact that they are helpful in only a fraction of symptomatic patients; many patients with arterial infections never have positive blood cultures. In the review by Brown and associates,19 only 60% of patients had positive preoperative blood cultures. In addition, blood cultures might not detect the infectious organism until several days or weeks have elapsed, limiting the test’s effect on clinical management.
The presence of bacteria in the blood may be an important early clue to an arterial infection, but the information from such tests must be evaluated in the proper clinical context. Most bacteremic patients have an evident source of bacteremia that should be identified and treated. Patients with positive blood cultures and no clinical evidence of a concurrent infection should be examined for possible arterial lesions. The significance of a positive blood culture in an otherwise asymptomatic patient is difficult to determine without considering the patient’s underlying problems and risk factors. It should also be noted that patients who are relatively asymptomatic (no systemic manifestations of sepsis) tend to have fewer positive blood cultures. As a result, in a study of patients undergoing clean arterial procedures, only 2% of blood cultures were positive, whereas 12% of arteries and 14% of periarterial adipose tissues harbored bacteria.28 Obvious clues, such as a recently noted aneurysm or a history of drug abuse, may promote further investigation.
Arterial Cultures
Because clinical decisions cannot always be based on arterial culture results, other techniques such as intraoperative Gram staining and frozen section of the arterial tissue are often considered. Unfortunately, these methods might not provide significant improvement in the detection of bacteria. In the study by Brown and associates,19 although 60% of patients had positive preoperative blood cultures, only 20% of intraoperative Gram stains were positive. Arterial wall frozen sections have not seen widespread use, but they may prove helpful. Histologic findings of inflammation and bacterial invasion are strong evidence supporting the diagnosis of arterial infection.
When obtaining blood or arterial wall cultures, it should be noted that the type of organism can affect the yield of the tests. Brown and associates19 noted that 60% of arterial wall cultures were negative. Approximately 25% of their cultures failed to detect any organism at all. Presumably, these were difficult organisms to collect and culture. S. epidermidis may be difficult to culture without sonicating the specimen. Treponema pallidum may require darkfield examination for identification. Mycobacterium tuberculosis is a fastidious organism that is difficult to grow. These considerations should prompt the special attention of the pathology laboratory and the collection of adequate specimens.
Molecular Diagnosis of Arterial Infection
Da Silva and colleagues reported use of PCR for detection of bacterial ribosomal nucleic acid (RNA) in specimens of normal aortic tissue removed at the time of surgical repair.29 Using universal eubacteria primers to amply 16SrRMA, they were able to identify the RNA from a wide variety of bacteria in the arterial specimen, thus demonstrating the potential of the technique. Their report is striking in its ability to detect bacteria that are ordinarily difficult to culture. Dickinson and colleagues30 reported the application of this technique in the case of a 72-year-old man with a suspected mycotic femoral artery aneurysm and blood cultures that showed S. pneumoniae. Arterial wall samples subjected to routine culture technique yielded no microorganisms. The same samples analyzed with pneumococcal primers for PCR were able to identify the presence of S. pneumoniae.
Angiography
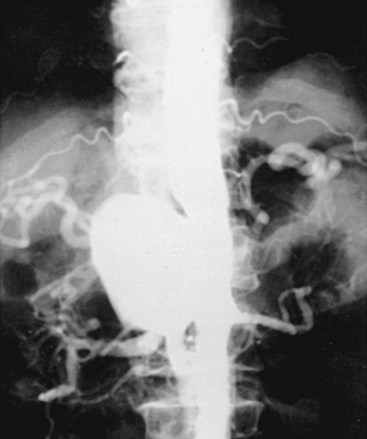
Angiography is the most widely used technique for the investigation and definition of arterial infections (Figure 10-4). Historically, it was the first method by which the characteristics of primary arterial infections were identified. Angiography served to define the characteristics of these lesions. Angiography is clearly superior in areas such as the intestinal mesentery and the visceral vessels, where the size of the arterial lesion may be less than the resolution of computed techniques. In the case of aortic mycotic aneurysms, the angiogram usually provides excellent definition of the defect in the aortic wall, the saccular pseudoaneurysm, and the contiguous arterial anatomy. Finally, the arteriogram offers the best definition of the relationship between the visceral vessels and the arterial defect—an essential step in planning patient management.
Hybrid Positron Emission Tomography and Computed Tomographic Scan
New “fusion technology” allows the images acquired from both a CT scanner and a PET scanner to be taken sequentially in the same session and combined into a single superposed or co-registered image. This allows combining the metabolic information of the PET scan with the spatial information of the CT scan. The technique may provide greater sensitivity and specificity in the detection and localization of an infection. Fukuchi and colleagues31 described their initial experience with this technique when applied to suspected vascular graft infections in 2005.31 They observed that this technique may be of particular benefit in patients who might have an infection; however, the presentation is not clinically evident or severe. Their experience indicated that the PET/CT had a high sensitivity and that in several instances this resulted in false-positive scans. In addition, the PET techniques suffer the general limitation of requiring the production of radiopharmaceuticals for the scan—a process that is highly specialized, requires significant infrastructure, and is expensive. This technique is still far from general clinical application.
Principles of Management
Control of Sepsis
The duration of antibiotic treatment is somewhat controversial, and several competing regimens have been proposed. Several authors suggest that IV antibiotics be initiated before surgery and extended for no less than 6 weeks postoperatively.32–34 In addition, these authors recommend that patients with prosthetic reconstructions, especially in situ prosthetic reconstructions, be prescribed lifelong oral regimens of suppressive antibiotics. Typically, oral trimethoprim-sulfamethoxazole (Bactrim), a sulfa drug, or a first-generation cephalosporin or penicillin is the agent of choice.
Nonoperative Therapy
Nonoperative therapy for arterial infections has been proposed by Kaufman and coworkers35 for high-risk or debilitated patients. This treatment modality, although effective anecdotally, remains controversial, and further investigation is necessary. Hsu and colleagues36 reported a series of 22 cases of infected aortic aneurysms treated with antibiotics only and without surgery.6 The in-hospital mortality was 50%. The event-free survival at 1 year was 32%. The authors noted that these results compared poorly to standard surgical approaches.
Arterial Reconstruction
Antibiotic-Impregnated Grafts.
Experimental and clinical evidence suggests that in situ reconstruction with antibiotic-impregnated prosthetic grafts is feasible. Bandyk and colleagues37 reported 27 patients operated on with rifampin-impregnated grafts as treatment for a variety of arterial infections. At least four recurrent infections were reported, including two that resulted in death. They concluded that in situ replacement using a rifampin-bonded prosthetic graft was most effective for low-grade staphylococcal arterial infection. An added caveat was that in the presence of virulent and antibiotic-resistant bacterial strains, this therapy usually failed. A more recent experience of 27 patients with both graft infections and mycotic aneurysms managed with silver-coated polyester grafts was published by Batt and associates.38 There were 7 perioperative deaths and 20 long-term survivors. Of the long-term (actuarial period, 24 months) survivors, one developed a recurrent infection. Rifampin may be more readily available rather than the silver-coated polyester grafts. Rifampin has been shown to have a significant effect in reducing in situ graft reinfection in experimental settings.39 This approach has been reported from a number of authors who note a low rate of recurrent infections (0% to 8%) involving the reconstruction graft when placed in situ.40–43
Vein Grafts.
Aortoilac reconstruction with autogenous veins has been performed with both saphenous and femoral veins. An initial experience using femoral veins for aortic and iliac reconstruction was reported by Schulman and associates.44 The use of superficial femoral and popliteal veins has been advocated by Clagett and associates45 for a number of infectious indications, including primary aortoiliac arterial infections has been termed the NAIS procedure. In their series of 38 patients with aortofemoral infections who underwent successful reconstruction with autogenous superficial femoral vein, the long-term primary patency was 85% at 5 years.45
Human Allografts.
Kieffer and associates46 reported their experience with 43 patients with infected infrarenal aortic prosthetic grafts who underwent in situ replacement using preserved allografts obtained from cadavers. In the early postoperative period, there were five septic complications, including two pseudoaneurysm ruptures; two cases of septic shock; and one instance of peritonitis from colon perforation. In follow-up, one late death may have been related to persistent infection. A report focusing on mycotic aortic aneurysms was published by Leseche and associates.47 They reported a series of 28 patients operated on for a variety of vascular infections, including five mycotic aneurysms. There were five perioperative deaths, two from sepsis and two from multisystem organ failure. The long-term outcomes were good. There were no recurrent infections and only three instances of aneurysmal degeneration of the allograft. More recent reports of cryopreserved human allograft indicate that the incidence of late aneurysmal degeneration may be further reduced by changes in cryopreservation processing.48–50
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree