Although the value of preventive cardiology may be more emphasized by general cardiovascular (CV) specialists as opposed to the interventional community, clearly secondary and even primary prevention guidelines are essential in the practice of interventional cardiology. Therefore, this chapter discusses many aspects of preventive cardiology, including some of the guidelines from the American Heart Association (AHA)/American College of Cardiology (ACC), as well as from many other major organizations.
AHA/ACC GUIDELINES
During the past decade, major guidelines have emphasized several different classes of medications in addition to therapeutic lifestyle changes (TLCs). Certainly, in interventional cardiology, antiplatelet therapy has been emphasized. In the absence of contraindications, most patients should receive aspirin for life and dual antiplatelet therapy (clopidogrel, prasugrel, ticagrelor) for at least 3 months and probably for 12 months in most patients following acute coronary syndrome (ACS) and percutaneous coronary intervention (PCI), which is discussed in detail elsewhere in this book. Lipid-lowering therapy (discussed in more detail below) is indicated to achieve a low-density lipoprotein cholesterol (LDL-C) of <100 mg/dL (<70 mg/dL is “optional” in high-risk patients). Therapy for hypertension (HTN) is indicated to achieve blood pressure (BP) levels of at least <140/90 mm Hg (lower levels are needed for certain patients as discussed below). In patients with diabetes mellitus (DM), hypoglycemic therapy is indicated to achieve near-normal fasting glucose levels (glycosylated hemoglobin, or HbA1C, <7%). Additionally, for most patients after myocardial infarction (MI) and with coronary heart disease (CHD), angiotensin-converting enzyme inhibitors (ACEIs) and β blockers (BBs) are indicated. In fact, the AHA developed the Get With The Guideline (GWTG) campaign during the last decade to increase adherence to many of these and other guidelines.
GENERAL CV RISK FACTOR INFORMATION
Dyslipidemia (DLP) and HTN produce a substantial burden in the United States and in most of westernized civilization (
1,
2,
3 and
4). A substantial number of deaths each year are estimated to be caused by systolic BP (SBP) higher than optimal (115 mm Hg in epidemiological studies) and by total cholesterol (TC) higher than optimal (estimated to be < 150 mg/dL). In fact, almost 60% of the CV disease burden is caused by BP and/or TC being higher than optimal. In the United States, concomitant HTN and DLP are highly prevalent, with HTN affecting nearly a quarter of the population, DLP impacting a third of the population, and both HTN and DLP impacting nearly 15%. Almost two-thirds of patients with HTN also have DLP, and nearly half of those with DLP have HTN. Many studies suggest that most patients with HTN and DLP are not at both goals.
Clearly, the risks for CHD events and strokes markedly increase with increasing age and increasing SBP. Likewise, there is a strong direct relationship between both TC and LDL-C and major CV and CHD events (
1,
3). Although both SBP and TC markedly increase overall risk, as seen with other risk factors, often the individual risk factors are more than additive, and actually potentiate each other in increasing overall risk, emphasizing the importance of multifactorial risk factor intervention (
1).
HYPERTENSION
The lifetime incidence of HTN has been increasing during recent decades and has now reached nearly 90% in the United States (
1). Approximately 70% of individuals with HTN are aware of their condition, and nearly 60% are receiving treatment. Nevertheless, HTN is controlled in only 30% of patients. Large meta-analyses show a nearly 50% increase in the long-term CV mortality for every 20 mm Hg increase in SBP above 115 mm Hg. Lowering elevated BP will lower risk of CV events regardless of age, race, gender, or other factors.
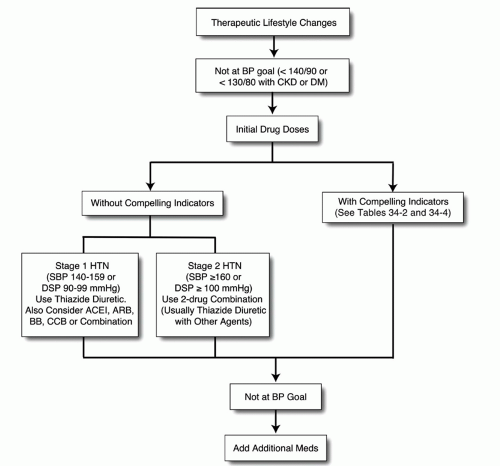
The recent HTN guidelines are based on the Joint National Commission (JNC) 7 recommendations. Ideal levels of SBP and diastolic BP (DBP) are <120 mm Hg and <80 mm Hg, respectively (
2). Prehypertension is considered a SBP of 120 to 139 mm Hg and/or DBP of 80 to 89 mm Hg. Stage 1 HTN is SBP of 140 to 159 mm Hg and/or DBP of 90 to 99 mm Hg. Stage 2 HTN is SBP of ≥ 160 mm Hg and/or DBP of ≥ 100 mm Hg, as summarized in
Table 34-1. TLCs are encouraged for all individuals, including those with prehypertension as well as documented HTN. Several classes of pharmacologic agents are indicated for stage 1 HTN, particularly the thiazide diuretics and ACEIs and a two-drug combination (usually a thiazide-type diuretic plus a second agent) for stage 2 HTN, as summarized in
Table 34-1.
In general, HTN therapy is recommended for BPs >140/90 mm Hg, but the current guidelines suggest BP treatment for patients with DM and/or chronic kidney disease (CKD) with BPs ≥130/80 mm Hg (
2). The target BP is at least under 140/90 mm Hg for all patients, but patients with CV disease may be treated to lower targets (
Fig. 34-1,
Tables 34-2 and
34-3) (
2,
5).
Although more intense BP treatment should be strongly considered in patients with DM, this remains controversial, because recent evidence has not noted better clinical event reduction when these patients are treated to levels of BP much below 140/99 mm Hg (
6). Likewise, in cohorts with CHD, more profound BP lowering may also induce potentially deleterious effects (
5).
In the last JNC recommendations, BBs are considered main agents for HTN, along with ACEIs, angiotensin-receptor blockers (ARBs), and calcium-channel blockers (CCBs). However, except for patients with heart failure (HF), postmyocardial infarction, and those with certain tachyarrhythmias, considerable evidence has questioned the routine use of BBs, especially with the older and nonvasodilating BBs, early in the course of HTN (
7). Other compelling indications for varying agents in the treatment of HTN are listed in
Table 34-3.
LIPID INTERVENTION
Substantial evidence from epidemiologic (trials of serial coronary angiography) and lipid intervention trials demonstrates the importance of TC, LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides on the development and progression of atherosclerosis and, especially, on the risk of major CV and CHD events (
1,
8,
9). Although levels of HDL-C and non-HDL-C actually correlate better with CHD than does LDL-C, the vast majority of the intervention data during the last two decades have focused on the role of LDL-C, which has been emphasized in the guidelines (
2,
10).
The National Cholesterol Education Program (NCEP)-Adult Treatment Panel (ATP) III treatment algorithm is based on LDL-C and assessing for CHD, CHD risk equivalents, and counting CHD risk factors (and assessing Framingham risk factor score in patients ≥2 risk factors;
Fig. 34-2) (
3). According to these guidelines, CHD risk equivalents are considered to be DM, as well as other forms of atherothrombotic disease such as peripheral arterial disease (PAD), abdominal aortic aneurysm, and symptomatic carotid disease, or having a Framingham risk score that suggests greater than a 20% 10-year risk of CHD. Certainly, from a clinical standpoint, many would consider other patients to have CHD risk equivalent, such as those with a high coronary artery calcium score, metabolic syndrome (MetS), or asymptomatic carotid disease (including those with a carotid bruit or a “significant” stenosis), but these are not “official” CHD risk equivalents based on NCEP-ATP III. As discussed with MetS and HTN, TLCs are always recommended by the treatment guidelines for lipids.
Most of the emphasis has been placed on the treatment of LDL-C and non-HDL-C (
Tables 34-4 and
34-5) (
10,
11 and
12). Although non-HDL-C has been in the NCEP-ATP III guidelines for over a decade, these have not been emphasized by practicing clinicians, who have largely focused on simply lowering LDL-C. However, once LDL-C goals are achieved, the guidelines state that the secondary objectives are to also achieve goals for non-HDL-C in patients who also have moderate elevations in TGs. In order to reduce non-HDL levels, one must either reduce LDL-C levels even further or direct therapy at lowering TGs/raising HDL-C. The one exception to this rule is for patients with severe hypertriglyceridemia (TGs ≥ 500 mg/dL), where primary therapy is lowering TGs and the secondary goal is lowering LDL-C and non-HDL-C.
From a pharmacologic perspective, the mainstay of DLP treatment is with statins, and recent evidence demonstrates the benefits of statins to reduce major CHD events, stroke, and mortality in both genders, despite the potential of statins to modestly increase the risk of new-onset type 2 DM (T2DM) (
12,
13,
14 and
15). Higher doses and more intensive statin therapy are more effective than lower doses in both improving lipid levels and reducing adverse CHD events, although higher doses have a greater effect on increasing risks for new-onset T2DM, and are more likely to cause subjective and objective side effects than do lower statin doses (
16). Statins are by far the most effective agents to reduce LDL-C, with the greatest potency noted with rosuvastatin, followed by atorvastatin, pitavastatin, simvastatin, and then the other agents (pravastatin, fluvastatin, and lovastatin) (
13). Other agents that reduce LDL-C are ezetimibe (which has limited clinical event data, especially in CKD), bile acid sequestrant resins, niacin and fibrates (
12).
As mentioned above, after LDL-C goals are achieved, the secondary goal focuses on non-HDL-C (
10,
12). In order to reduce non-HDL-C levels, therapy can be expanded to include efforts at lowering TGs with fibrates, niacin, or high-dose omega-3 fatty acids (fish oils), or increasing levels of HDL-C with fibrates and niacin (
Fig. 34-3) (
12). Although many trials over the years have supported therapy directed at TGs and HDL-C, recent trials on these topics have disappointing results (
17,
18).