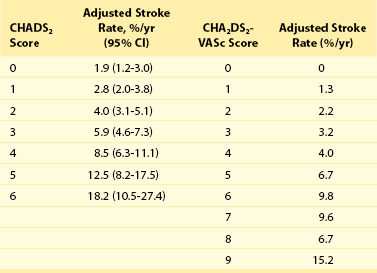
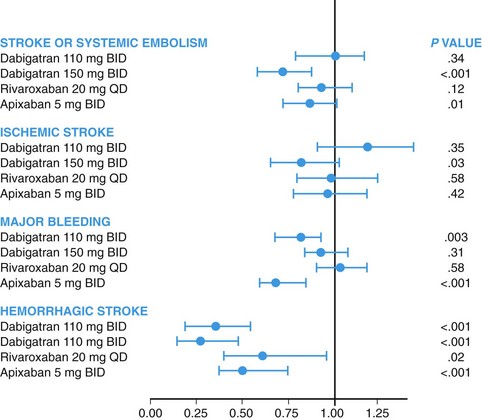
114 Patients with atrial fibrillation have a fivefold increased risk of stroke, and one sixth of all strokes are attributable to AF.1 Moreover, strokes complicating AF are often large and clinically disabling.2 The rate of systemic embolism or stroke in patients with AF varies greatly between patients, ranging from less than 1% to over 10% per year, depending on the presence of additional risk factors. Therefore, clinical decision making regarding stroke prevention in patients with AF must incorporate risk stratification. Many risk factor systems have been developed to facilitate the evaluation of patients with AF. The CHADS2 score is the most widely used and validated. It is based on a point system in which two points are assigned for a history of stroke or transient ischemic attack, and one point is assigned for any of the following risk factors: congestive heart failure, systemic hypertension, age greater than 75 years, and diabetes mellitus (Table 114-1). The annual risk of stroke increases with increasing score (see Table 114-1). The CHA2DS2-VASc score gives additional points for the following risk factors: age between 65 and 75 years, female sex, and presence of vascular disease (defined myocardial infarction, complex aortic plaque, or peripheral artery disease). This risk stratification scheme has been recommended by some international guidelines and is useful to patients with a CHADS2 score of 1 where there is often uncertainty about whether or not the risk of stroke is sufficiently high to justify the use of anticoagulation. The CHA2DS2-VASc score of patient with a CHADS2 score of 1 can vary from 1 to 4, with ischemic stroke risk increasing significantly throughout this range of scores.3 All current guidelines recommend the routine use of CHADS2 or CHA2DS2-VASc score in patients with AF. Bleeding is the major adverse effect of oral anticoagulant (OAC) therapy. Bleeding risk varies by patient, and it should be assessed carefully before starting anticoagulant therapy. Several validated bleeding risk scores have been developed to quantitate this risk, of which the HAS-BLED is currently the most popular. The score is based on a point system in which one point is assigned for any of the following: hypertension, abnormal renal or liver function, stroke, bleeding history or predisposition, labile INR, old age, and concomitant use of drugs or alcohol. The annual risk of bleeding increases as the risk score is higher. Scores of 3 or greater indicate high risk, requiring caution and regular review following the initiation of antithrombotic therapy4 (Table 114-2). Table 114-2 *Systolic blood pressure > 160 mm Hg. †Chronic dialysis, renal transplantation, serum creatinine ≥ 200 µmol/L. ‡Chronic hepatic disease (e.g., cirrhosis) or biochemical evidence of significant hepatic derangement (bilirubin > 2 x upper limit of normal in association with AST/ALT > 3 x upper limit normal). §Concomitant use of drugs such as antiplatelet agents, nonsteroidal antiinflammatory drugs. Several trials have evaluated aspirin and other antiplatelet drugs for stoke prevention in AF. A metaanalysis of these trials suggests that aspirin reduces the risk of stoke by 22% (95% confidence interval [CI], 0.02 to 0.38).5 Recently, some authors have questioned whether aspirin is truly effective against stroke in AF, based on an apparent lack of efficacy in at least one large cohort study.6 Because of the greater relative efficacy of warfarin and other oral anticoagulants, the role of aspirin is diminishing. Two trials have evaluated the effectiveness and safety of the combination of aspirin and clopidogrel for the prevention of stroke in patients with AF. ACTIVE-W was designed to compare the effects of clopidogrel (75 mg/day) in addition to aspirin (75 to 100 mg/day) compared to warfarin (target INR, 2.0 to 3.0) in patients with AF with at least one additional risk factor for stroke. The study was terminated early because of the clear superiority of warfarin. Patients who received warfarin had lower rates of primary outcomes (stroke, systemic embolism, myocardial infarction, or vascular death; annual risk of 3.9% and 5.6%, respectively; relative risk [RR], 1.44; 95% CI, 1.18 to 1.76; P = .0003). The main effect was derived from a significant reduction of stroke (RR, 1.72; 95% CI, 1.24 to 2.37; P = .001) and systemic embolism (RR, 4.66; 95% CI, 1.58 to 13.8; P = .005) related to warfarin use. Furthermore, there were no differences in major bleeding between groups (2.42% per year with clopidogrel plus aspirin vs. 2.21% per year with warfarin; RR 1.1; 95% CI, 0.83 to 1.45; P = .53).7 ACTIVE-A, a double-blind randomized trial, compared clopidogrel (75 mg/day) plus aspirin (75 to 100 mg/day) to aspirin alone in patients with AF at risk for stroke but who were unsuitable for therapy with vitamin K antagonists (VKAs). The combined antiplatelet therapy was demonstrated to be superior to aspirin alone, with a 28% reduction in stroke (RR, 0.72; 95% CI, 0.62 to 0.83; P < .001). Dual antiplatelet therapy, however, was associated with higher risk of major bleeding (RR, 1.57; 95% CI, 1.29 to 1.92; P < .001).8 Oral anticoagulation therapy with VKAs has been the gold standard for stroke reduction in AF since the early 1990s. At that time, several randomized controlled trials demonstrated that warfarin and other VKAs reduce the risk of stroke in AF. In a metaanalysis of the trials of VKAs compared with placebo or usual care, the relative risk reduction was 62% (95% CI, 0.48 to 0.72).5 Some trials have compared warfarin to aspirin, and the metaanalysis of these shows that warfarin reduces the risk of stroke (RRR, 36%; 95% CI, 0.14 to 0.52).5 The benefit of warfarin comes with an increased risk of hemorrhage. In a comparison with aspirin, the metaanalysis showed that warfarin doubles the risk of major extracranial hemorrhage (RR, 2.0; 95% CI, 1.2 to 3.4; absolute risk increase, 0.2% per year) and intracranial hemorrhage (RR, 2.1; 95% CI, 1.0 to 4.6).5 Warfarin has been widely recognized as effective and relatively safe for stroke prevention in AF. Despite these clearly demonstrated effects and strong recommendations by international guidelines, one can estimate that approximately 50% to 60% of patients at risk for stroke and without major contraindications receive long-term VKA. Rates of use like vary greatly globally, with even lower rates of use in many countries. Rates of discontinuation of VKA therapy are high, with approximately half of patients discontinuing therapy within 3 to 5 years. Even for those receiving therapy, in many settings poor control of the INR reduces the effectiveness of therapy.9–13 The underpresciption of warfarin is in large part related to the many limitations of VKA therapy, which include the narrow therapeutic window, the need for rigorous and inconvenient INR monitoring, the many interactions with food and other drugs, the need for discontinuation and periprocedural bridging with heparin, and the genetic variability in metabolism. These limitations have prompted the development of more convenient alternatives to VKAs. Several oral drugs directly inhibiting either coagulation factor II (thrombin) or factor Xa have been developed as alternatives to warfarin for stroke prevention in AF (see Table 114-2). Dabigatran etexilate is orally administrated and rapidly converted to dabigatran by esterases. The bioavailability is 6% to 7% and it has onset of action within 2 hours of administration. The elimination half-life is 12 to 14 hours, and approximately 80% of the drug is excreted unchanged by the kidneys.14 Dabigatran is approved worldwide for prevention of stroke in AF.14 The safety and efficacy of dabigatran was assessed in the RE-LY trial, an 18,000-patient noninferiority multicenter randomized clinical trial that compared two different blinded doses of dabigatran to unblended dose-adjusted warfarin (INR target of 2.0 to 3.0, achieved 64% of the time). Eligible patients had at least one risk factor for stroke in addition to AF and had an estimated creatinine clearance of 30 mL/min or greater. Dabigatran (150 mg) was shown to be superior to warfarin in reducing the primary outcome, which was stroke (including hemorrhagic) and systemic embolism by 34% (P < .001) with no significant difference in major bleeding. Compared with warfarin, dabigatran (110 mg) was noninferior to warfarin in preventing stroke, and systemic embolism and was associated with a 20% relative risk reduction in major bleeding (P = .003) (Figure 114-1). Both dosages of dabigatran lowered the risk of hemorrhagic stroke and subdural hemorrhage, with highly significant reductions of greater than 50%. Gastrointestinal major bleeding was more common with higher dose dabigatran than warfarin, and there was a trend for higher rates of myocardial infarction with dabigatran than with warfarin. Figure 114-1 Cumulative hazard rates for the primary outcome of stroke or systemic embolism, according to treatment group. Dabigatran has important advantages over warfarin, including large reduction in intracranial bleeding for both doses, reduction in ischemic stroke with the higher dose, and reduction in major bleeding with the lower dose. The major international guidelines now recommend dabigatran for stroke reduction in AF. The higher dose of dabigatran is recommended for many patients, in view of its superior efficacy in the prevention of stroke and systemic embolism. However, the lower dose of dabigatran is recommended in some countries where there is a higher risk of bleeding (HAS-BLED score ≥ 3), moderate renal impairment (creatinine clearance, 30 to 49 mL/min) and in most countries the elderly (age ≥ 80 years) because of an increase in extracranial bleeding with age on both doses of dabigatran.15 The lower dose of dabigatran is not available in the United States. The noninferiority of rivaroxaban compared with warfarin was demonstrated in the ROCKET-AF study, which was a randomized, double-blind, double-dummy, event-driven trial in which 14,264 patients with AF and a CHADS2 score of 2 or greater were randomly assigned to receive 20 mg rivaroxaban once daily (15 mg in cases of creatinine clearance between 30 and 49 mL/min) or dose-adjusted warfarin (INR target of 2.0 to 3.0, achieved 58% of the time). Noninferiority on efficacy was achieved with similar rates of major and nonmajor clinically relevant bleeding events compared with warfarin. There was a large reduction in intracranial hemorrhage, and there was a reduction in fatal bleeding.16 Several guidelines currently recommend rivaroxaban as an alternative to warfarin. The dose of 20 mg is used for most patients, with the 15-mg dose being used for patients with moderate renal impairment (creatinine clearance, 30 to 49 mL/min).15 Apixaban has a half-life of approximately 12 hours and is administrated twice daily. The metabolism is 25% renal, and bioavailability is high. Similar to rivaroxaban, cytochrome P450 is involved with metabolism so that strong inhibitors substantially increase drug levels.17 Two large, randomized, double-blind trials have been conducted with apixaban for stroke prevention in AF. The AVERROES compared the efficacy of apixaban (5 mg twice daily) with aspirin (81 to 324 mg once daily) for prevention of stroke and systemic embolism in 5599 patients with AF who were considered unsuitable for VKA treatment. There was a greater than 50% reduction in stoke or systemic embolism with apixaban (RR, 0.46; 95% CI, 0.33 to 0.64; P < .001). Although minor bleeding was more common in the apixaban group, the rates of major, fatal, and intracranial bleeding did not differ between the groups. Apixaban was better tolerated than aspirin, with significantly fewer study drug discontinuations.18 Apixaban was also compared with warfarin in the ARISTOTLE study, a randomized, double-blind trial for the prevention of stroke and systemic embolism in 18,201 subjects with nonvalvular AF and at least one additional risk factor for stroke. Compared to warfarin, apixaban reduced stroke and systemic embolism by 21% (P = .01), bleeding by 31% (P < .001), and mortality by 11% (P = .047). Apixaban was well tolerated and resulted in less drug discontinuation.19 Some guidelines have recommended apixaban as an alternative to warfarin already, anticipating future approvals. Table 114-3 shows a comparison of the pharmacologic characteristics of warfarin and the new oral anticoagulants for atrial fibrillation. Figure 114-2 summarizes the results of the main randomized clinical trials of new anticoagulants on stroke prevention in patients with AF.
Prevention of Stroke in Patients With Atrial Fibrillation
Stroke Risk Stratification of Patients With Atrial Fibrillation
Thromboembolic Risk Scores
Bleeding Risk
Letter
Characteristics
Points
H
Hypertension*
1
A
Abnormal renal† and liver‡ function (1 point each)
1 or 2
S
Stroke
1
B
Bleeding
1
L
Labile INR
1
E
Elderly (>65 years old)
1
D
Drugs§ or alcohol (1 point each)
1 or 2
Antithrombotic Therapy
Aspirin
Combination of Aspirin and Clopidogrel
Vitamin K Antagonist Therapy for Stroke Prevention in Atrial Fibrillation
Novel Oral Anticoagulants
Thrombin Inhibitors: Dabigatran
Xa Inhibitors
Rivaroxaban
Apixaban
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Prevention of Stroke in Patients With Atrial Fibrillation