Mechanisms of acute myocardial infarction and nonobstructed coronary arteries (MINOCA) are incompletely understood. Myocardial bridging (MB) is usually considered a benign congenital variant, but serious complications have been reported. MB has also been proposed as a cause of takotsubo syndrome (TS). We aimed to examine whether MB was more frequent in patients with MINOCA or TS than in age- and gender-matched controls and to compare the MB detection rates of coronary computed tomography angiography (CTA) and invasive coronary angiography (ICA). Patients with MINOCA (n = 57) and age- and gender-matched controls (n = 58), aged 45 to 70 were enrolled. Myocarditis was excluded by cardiovascular magnetic resonance imaging. Patients with TS (n = 15) were considered as a subgroup and therefore not excluded. Patients with MINOCA underwent ICA and all study participants underwent coronary CTA. All examinations were reviewed with focus on MB. Among 57 MINOCA patients, 15 MINOCA patients with TS and 58 controls, MB was demonstrated in 28 patients (49%), 8 patients (53%), and 26 patients (45%), respectively. There were no statistically significant differences regarding the prevalence of MB or the type, location, length, or thickness of MB. There was a statistically significant difference (p <0.01) between the detection rates of coronary CTA and ICA that demonstrated MB in 54 subjects (47%) and 13 subjects (23%), respectively. In conclusion, MB was frequent with a similar prevalence in patients with MINOCA, patients with TS and controls. This suggests that there is no causal link between MB and MINOCA or TS. Furthermore, coronary CTA detects MB at a significantly higher rate than ICA.
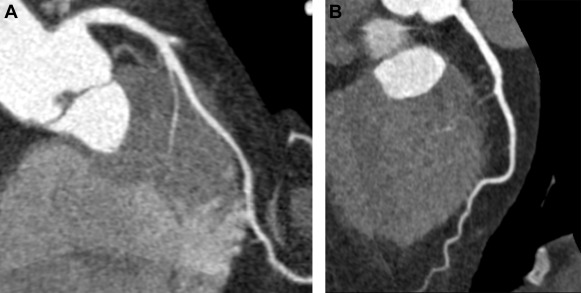
The term “myocardial bridging” (MB) refers to a condition in which a segment of an epicardial coronary artery (the “tunneled segment”) is covered by cardiac muscle fibers (the “myocardial bridge”), as shown in Figure 1 . The reported prevalence of MB varies greatly: 15% to 85% at autopsy, 0.5% to 4.5% at invasive coronary angiography (ICA) and 26% to 58% at coronary computed tomography angiography (CTA). Most MBs can be considered as benign congenital variants. However, serious adverse events such as myocardial ischemia and sudden death have been reported. Underlying mechanisms for myocardial infarction and nonobstructed coronary arteries (MINOCA) and takotsubo syndrome (TS) are questions of debate. A recent study suggested that MB might have a causative role in TS. Our aim was to examine whether MB could be a mechanism for MINOCA or TS, by comparing the prevalence of MB detected by coronary CTA in patients with MINOCA (with or without TS) and in a control group, matched by age and gender. The secondary aim was to study the MB detection rate of ICA compared to that of coronary CTA.
Methods
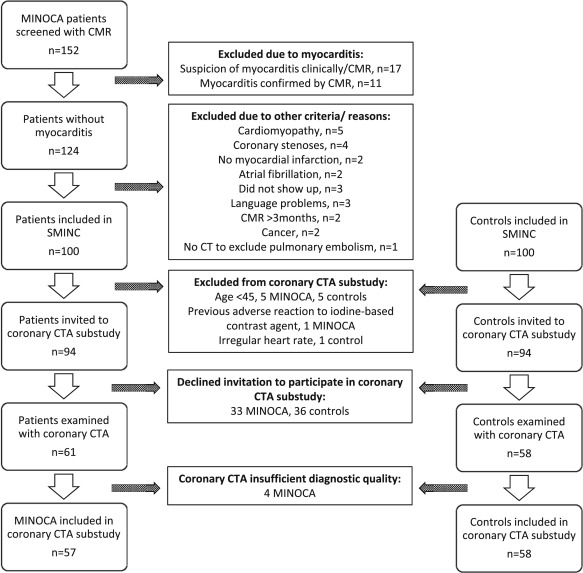
The present study is a substudy of the Stockholm Myocardial Infarction with Normal Coronaries (SMINC) study. The study conforms to the principles of the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Stockholm and by the Radiation Protection Committee at the Karolinska University Hospital. Written informed consent was obtained from all study participants. The patients with MINOCA and the controls of the present study form a subgroup of the study participants of the SMINC study, as previously described. Briefly, patients with MINOCA were screened for the SMINC study at 5 different coronary care units in the Stockholm metropolitan area from June 2007 to May 2011. Patients were eligible to take part in the study if they were aged between 35 and 70 years, fulfilled the criteria for acute myocardial infarction (MI) according to the universal definition of MI, and underwent ICA showing no or minimal signs of atherosclerosis (no stenosis exceeding 30% by visual estimation). All patients also underwent cardiovascular magnetic resonance (CMR) imaging. Exclusion criteria were myocarditis, a clinical diagnosis of pulmonary embolism, non sinus rhythm on admission, pacemaker use and a patient history of heart disease, chronic obstructive lung disease, or renal disease. After patient inclusion, the coronary angiogram and the clinical diagnosis of acute MI were reevaluated by an additional independent investigator. Patients with TS, according to the Mayo Clinic diagnostic criteria, were considered part of the MINOCA group and thus not excluded. The screening process, including CMR imaging protocols, analyses, and results, has been described in a previous publication. A control group, matched by age and gender, was recruited using a registry comprising all Stockholm residents. Randomly selected subjects of matching age and gender to patients with MINOCA received a letter of invitation followed by a telephone call. Subjects who were willing to participate and who had no known cardiovascular disease underwent an exercise stress test. If the test was normal, they were invited to take part in the study. Of 152 patients screened with CMR for the SMINC study, 100 patients with MINOCA and 100 controls were, after exclusions described previously, invited to take part in a coronary CTA substudy. Additional exclusion criteria for the CTA substudy were age <45, previous adverse reaction to an iodine-based contrast agent, and an irregular heart rate. Patient selection and study flow are shown in Figure 2 .
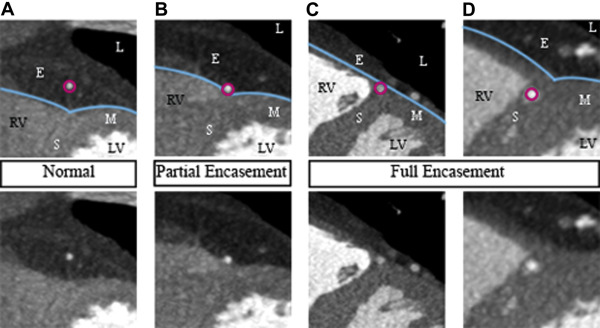
Examinations were performed on a 64-slice computed tomography (CT) scanner (LightSpeed VCT XT; GE Healthcare, Milwaukee, Wisconsin). A prospectively electrocardiogram-triggered scan protocol was used: detector configuration 64 × 0.625 mm, rotation time 350 ms, tube potential 120 kVp, tube current 450 to 650 mA, and padding 0 to 200 ms. The contrast agent used was iodixanol 320 mg I/ml (Visipaque; GE Healthcare, Stockholm, Sweden), individually dosed on the basis of body weight (400 mg I/kg, 75 to 100 ml iodixanol) and administered using a dual-head injector (Medrad, Stellant Dual Head Injector; Pittsburgh, Pennyslvania) and a triple-phase protocol. In the absence of contraindications, patients received metoprolol (25 to 100 mg) per os before the examination and sublingual nitroglycerine (0.4 mg) 4 minutes before the scan. The coronary CTA examinations were analyzed by 2 experienced readers (level 2 according to the American College of Cardiology Foundation/American Heart Association levels of competence ), who were blinded to all clinical information. Coronary CTA analysis was performed using the CardIQ Xpress software on the Advantage Workstation 4.4 (GE Healthcare, Milwaukee, Wisconsin). Axial source images, multiplanar and curved multiplanar reformats, and thin-slab maximum intensity projections were used. The optimal image display setting was chosen on an individual basis (in general at a window width of 800 to 1,000 HU and a level of 100 to 200 HU). Coronary arteries were subdivided into 17 segments, according to the modified American Heart Association classification. Initially, each segment was assessed regarding image quality and evaluability. Segments were considered non evaluable if artifacts prevented reliable assessment of the lumen or the vessel wall. Second, a clinical reading was performed, evaluating the coronary CTA examinations with regard to the presence and degree of stenosis as well as plaque size and composition. Finally, an additional joint reading was performed with focus on MB. The presence of MB was noted and defined as MB with partial encasement or full encasement, as described by Kim et al. MB was thus defined as a coronary artery segment being in direct contact with the myocardium (partial encasement) or surrounded by myocardium (full encasement), instead of being surrounded by epicardial fat as it normally would ( Figure 3 ). In the case of 1 MB involving 2 adjacent coronary segments, the MB was attributed to the more proximal segment. The length of the MB was measured on multiplanar reformats and, in the case of full encasement, the thickness of the overlying myocardial bridge was measured in a short-axis view, perpendicular to the tunneled segment.
All patients with MINOCA underwent ICA at the time of initial hospital admission, according to clinical routines and using standard techniques, including a transfemoral or transradial approach and a minimum of 2 different projections of each coronary artery. After an initial clinical assessment at the hospitals where the patients were admitted, there were additional joint readings by 2 experienced interventional cardiologists, performing careful reviews of the coronary angiograms and focusing specifically on the presence of MB. Blinded to the findings of coronary CTA, they assessed indirect signs of MB, that is, systolic compression of the tunneled segment or a localized change in direction of the vessel course toward the ventricle (also described as the step-down–step-up phenomenon). As for the coronary CTA readings, coronary arteries were subdivided into 17 segments.
Initially, data were characterized using descriptive statistics and assessed for normal distribution using the Shapiro–Wilk test. Categorical variables are presented as absolute value and percentage and were compared using the chi-square test or Fisher’s exact 2-sided test. Continuous variables with a normal distribution are presented as mean ± SD and non normally distributed continuous variables are presented as median (range). Groups were compared using the independent samples Student t test for normally distributed variables or the Mann–Whitney U test in the case of non normal distribution. All analyses were carried out using IBM SPSS Statistics version 22.0. The 5% level of significance was considered.
Results
Coronary CTA was performed in 119 (61 patients with MINOCA + 58 controls) study participants. However, 4 CT examinations of patients with MINOCA were excluded; 3 because of motion artifacts resulting in <7 evaluable segments and 1 because of the heart not being completely covered by the scan. Thus, cardiac CT exams of 57 patients with MINOCA and 58 controls were further analyzed. In total 1,574 coronary segments were identified. Of these 15 individual segments (1.9%) in the MINOCA group and 11(1.4%) in the control group were non evaluable and excluded from analyses. All patients with MINOCA underwent ICA. Baseline characteristics of patients with MINOCA and controls are compared in Table 1 . Fifteen patients (26%) with MINOCA received a final diagnosis of TS. A detailed report on the coronary artery plaque burden, as determined by coronary CTA, in patients with MINOCA and in controls has been published. Briefly, CAD was demonstrated in a large proportion (58%) of the MINOCA group, despite the normal or near-normal coronary angiogram and in 57% of controls. As expected, there were no patients with MINOCA with significant CAD (defined as ≥50% luminal diameter reduction).
| Variable | MINOCA patients (n=57) | Control group (n=58) | p-value |
|---|---|---|---|
| Age (years) | 60 ± 5 | 61 ± 6 | Ns |
| Female | 42 (74%) | 39 (67%) | Ns |
| Body mass index (kg/m 2 ) | 25.8 ± 3 | 25.8 ± 3 | Ns |
| Current smoker | 10 (18%) | 2 (3%) | <0.05 |
| Prior smoker | 17 (30%) | 23 (40%) | Ns |
| Diabetes mellitus | 1 (2%) | 0 | Ns |
| Treated hypertension | 19 (33%) | 6 (10%) | <0.01 |
| Treated hyperlipidemia | 8 (14%) | 3 (5%) | Ns |
| Family history of coronary artery disease | 16 (28%) | 14 (24%) | Ns |
Only 3 study participants had MB (as determined by coronary CTA) in vessels other than the left anterior descending artery (LAD). Owing to the vast majority of MBs being located in the LAD, only these were included in further analyses. Table 2 provides the prevalence, type, and location of MB in the different study groups. In total, 54 study participants (47%) had evidence of MB. MB with full encasement was found in 33 subjects (29%), and MB with partial encasement was found in 21 subjects (18%). A majority of MBs were located in segment 7 (mid LAD). There were no statistically significant differences between patients with MINOCA or the TS subgroup and the control group regarding the prevalence or type of MB. Nor were there any differences between the groups regarding the location, the length, or the thickness of MB ( Table 2 ). There were 9 patients with MINOCA with no signs of CAD or MB. Of these 9 patients, 3 were diagnosed with TS.
| MINOCA patients (n=57) | TS subgroup (n=15) | Control group (n=58) | p-value | |
|---|---|---|---|---|
| Myocardial bridging (coronary CTA) | 28 (49%) | 8 (53%) | 26 (45%) | ns |
| Type of Myocardial bridging | ns | |||
| Partial encasement | 11 (19%) | 2 (13%) | 10 (17%) | |
| Full encasement | 17 (30%) | 6 (40%) | 16 (28%) | |
| Location of myocardial bridging | ns | |||
| Segment 6 | 1 (2%) | 1 (7%) | 0 | |
| Segment 7 | 23 (40%) | 7 (47%) | 20 (34%) | |
| Segment 8 | 4 (7%) | 0 (0%) | 6 (10%) | |
| Length of myocardial bridging (mm) | 14.5 (2.0-51.0) | 7.3 (2.0-10.9) | 13.5 (3.8-45.0) | ns |
| Thickness of myocardial bridging (mm) ∗ | 0 (0-4.8) | 0 (0-2.0) | 0 (0-5.0) | ns |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


