Limited amount of data suggest that patients with aortic stenosis and pulmonary hypertension (PH) who undergo transcatheter aortic valve replacement (TAVR) experience decrease in PH postprocedure. Inconsistent use of systolic pulmonary artery pressure cut-off values in previous studies limits our ability to draw meaningful conclusions regarding the prognostic role of PH in assessment of TAVR candidates. A total of 415 consecutive patients who underwent TAVR were included in the present study. Two groups were compared based on receiver-operating characteristics curve analysis for the best SPAP value to predict outcome, yielding 2 study groups of no/mild PH (≤50 mm Hg; n = 172, 41%) versus moderate/severe PH (>50 mm Hg; n = 243, 59%). Demographics and co-morbidities were comparable between the 2 groups; however, right-sided cardiac failure (35% vs 19.8%, p = 0.02) and mitral regurgitation (18.4% vs 8.6%, p = 0.007) were more frequent in patients with moderate/severe PH. Procedural characteristics and complications were comparable between the groups. Although there was an early overall decrease in SPAP postprocedure, only 26% of moderate/severe patients with PH experienced a significant decrease in SPAP (>10 mm Hg). The 30-day (14.5% vs 7.4%, p = 0.02) and 1-year mortality (30.8% vs 21%, p = 0.02) was higher in moderate/severe patients with PH. In multivariate analysis, systolic pulmonary artery pressure and chronic lung disease were identified as independent predictors for mortality at 1 year. PH is a frequent co-morbidity in patients with severe aortic stenosis who underwent TAVR. Significantly elevated pulmonary artery pressures at baseline may serve as a poor prognostic factor when performing preprocedural assessment of the patients.
Historically, presence of pulmonary hypertension (PH) as a co-morbidity in patients with severe aortic stenosis (AS) who undergo aortic valve surgery was considered as a predictor for poor outcome. This increased risk was linked to hemodynamic challenges during the operative and postoperative period related to the use of cardiopulmonary bypass and because of pulmonary vasculopathy and right-sided cardiac failure.
Transcatheter aortic valve replacement (TAVR) might offer several advantages for patients with severe AS and PH because the procedure is performed without major hemodynamic changes that are typically associated with cardiopulmonary bypass and mechanical ventilation, which are not mandatory during TAVR. Limited amount of data suggest that patients with AS and PH who undergo TAVR experience decrease in PH postprocedure. However, patients with PH still have worse prognosis compared with patients without PH. Furthermore, data regarding the long-term hemodynamic effects of TAVR in patients with PH are limited. Thus, the goals of the present study were to assess the prevalence of PH in patients with AS who undergo TAVR and to evaluate the long-term effects on pulmonary artery hemodynamics after TAVR.
Methods
The study was approved by the Institutional Review Board of the MedStar Health Research Institute. Consecutive patients with symptomatic severe AS who underwent TAVR from 2007 to 2013 at MedStar Washington Hospital Center were analyzed. Prespecified clinical and laboratory data were collected for all patients at baseline before the procedure, immediately postprocedure, during the index hospitalization, and up to 1 year. Collected data included medical history, electrocardiogram, echocardiographic studies, laboratory tests, and clinical outcomes.
Patients underwent TAVR either with the balloon-expandable Edwards SAPIEN or the SAPIEN-XT transcatheter heart valves (Edwards Lifesciences, Irvine, California) or the self-expandable Medtronic CoreValve (Medtronic, Minneapolis, Minnesota) through the transfemoral, transapical, or direct aortic access routes as part of PARTNER, US, CoreValve studies, or standard clinical care, as previously described. Doppler tracings and 2-dimensional images were obtained from parasternal long- and short-axis, apical 4-chamber, and subcostal 4-chamber views. Transthoracic echocardiograms were reviewed to assess the pericardium, valvular anatomy and function, and cardiac function. Tricuspid regurgitant flow was identified by color flow Doppler techniques. Continuous-wave Doppler measured maximum jet velocity. Right ventricular systolic pressure was estimated based on the modified Bernoulli equation and was considered equal to the systolic pulmonary artery pressure (SPAP) in the absence of right ventricular outflow obstruction. SPAP was calculated by adding transtricuspid pressure gradient to mean right atrial pressure estimated from inferior vena cava diameter and motion during respiration as follows: if the caliber of inferior vena cava was normal (1.5 to 2.5 cm), mean right atrial pressure was estimated to be 5 mm Hg if there was complete collapse of the inferior vena cava during inspiration or was estimated to be 10 mm Hg if the inferior vena cava collapse was >50%. If the inferior vena cava was dilated, mean right atrial pressure was estimated to be 15 mm Hg if the inferior vena cava collapsed by <50% with inspiration or was estimated to be 20 mm Hg if there was no visible evidence of inferior vena cava collapse. Right ventricular function was assessed according to the American Society of Echocardiography guidelines using the tricuspid annular plane systolic excursion method.
Right-sided heart catheterization was performed with a 7Fr Swan-Ganz catheter in 199 of the patients (48%). Right atrial pressures (amplitude of a and v waves and mean pressure), right ventricular systolic and diastolic pressures, pulmonary artery pressures (systolic, diastolic, and mean), and pulmonary capillary wedge pressures (a and v waves and mean pressure) were measured. Cardiac output was determined by the thermodilution method.
Inhospital outcomes were collected according to the Valve Academic Research Consortium-2 consensus document. Out-of-hospital adverse events were assessed up to 1 year by means of outpatient clinic visit or a standardized telephone interview. All suspected events were adjudicated by a blinded interventional cardiologist.
Statistical analysis was performed using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina). Continuous variables are expressed as mean ± SD or median (twenty-fifth to seventy-fifth interquartile range) as appropriate according to variable distribution. Categorical variables are expressed as percentages. Student’s t test was used to compare continuous variables, and the chi-square test or Fisher’s exact test was used to compare categorical variables. Pearson’s correlation coefficients were used to assess correlation between pulmonary pressure measurements by echocardiography and right-sided cardiac study.
Receiver-operating characteristic (ROC) curve was analyzed to assess the discriminatory ability of baseline SPAP to predict 30-day mortality and to determine the best cut-off value to predict this end point. For that purpose, the best prognosticator in ROC curve analysis was considered to be the value that gave the highest product of sensitivity and specificity for predicting 30-day mortality.
Survival rates up to 1 year were computed using the Kaplan-Meier method, and differences in parameters were assessed using the log-rank test. A Cox proportional hazards analysis was performed to assess the independent influence of baseline PH on 1-year mortality in each group. Variables of the baseline and clinical characteristics associated with the outcome of interest were adjusted in the multivariable analysis: age, previous myocardial infarction, chronic renal failure, left ventricular ejection fraction, Society of Thoracic Surgeons (STS) score, chronic lung disease, and peripheral vascular disease. A p value <0.05 was considered statistically significant.
Results
A total of 415 patients were included in the present study. The average age was 84 ± 8 years, and 47% of the patients were men. The study population comprised a high-risk patient population with an average STS risk score of 10 ± 5 and Logistic euroSCORE of 30 ± 24. A large proportion of the patients had significant co-morbidities, such as diabetes (31%), chronic lung disease (31%), renal insufficiency (55%), previous coronary bypass surgery (31%), and left ventricular ejection fraction <40% (23%).
By ROC curve analysis, baseline SPAP demonstrated intermediate prognostic power as a single parameter to predict 30-day mortality (area under the curve 0.62; Figure 1 ). Based on these findings, an SPAP cut-off value of 50 mm Hg was selected to predict 30-day mortality for patients who underwent TAVR, yielding sensitivity and specificity of 57% and 60%, respectively. This cut-off value was used to determine the study groups of no/mild PH (≤50 mm Hg) versus moderate/severe PH (>50 mm Hg). Most patients were included in the moderate/severe PH group (243 of 415, 59% of the entire cohort).

Overall, as listed in Table 1 , baseline characteristics and co-morbidities of patients in both groups were comparable between patients with no/mild versus moderate/severe PH.
| Variable | Pulmonary hypertension | p Value | |
|---|---|---|---|
| No/mild (n=172) | Moderate /severe (n=243) | ||
| Age (years±SD) | 83±8 | 84±8 | 0.08 |
| Men | 116 (48%) | 80 (47%) | 0.8 |
| Risk assessment | |||
| Society of Thoracic Surgeons score risk (%±SD) | 9.9±4.5 | 10.2±5 | 0.6 |
| Log EuroSCORE risk (%±SD) | 31±23.6 | 28.3±24.9 | 0.4 |
| Co-morbidities | |||
| Systemic hypertension | 218 (94%) | 152 (92%) | 0.5 |
| Diabetes mellitus | 69 (30%) | 55 (33%) | 0.5 |
| Chronic obstructive lung disease | 73 (31%) | 53 (33%) | 0.8 |
| Renal failure | 119 (53%) | 95 (58%) | 0.3 |
| Hemodialysis | 4 (1.8%) | 4 (2.4%) | 0.7 |
| Peripheral vascular disease | 86 (38%) | 51 (32%) | 0.3 |
| History of coronary artery disease ∗ | 137 (78%) | 95 (75%) | 0.5 |
| Prior myocardial infarction | 48 (21%) | 23 (14%) | 0.09 |
| Prior percutaneous coronary intervention | 76 (33%) | 44 (27%) | 0.2 |
| Prior coronary artery bypass surgery | 76 (33%) | 48 (29%) | 0.5 |
| Prior valve surgery | 5 (2.8%) | 6 (4.4%) | 0.5 |
| Atrial fibrillation | 92 (40%) | 73 (44%) | 0.4 |
| Prior stroke or transient ischemic attack | 43 (19%) | 28 (18%) | 0.7 |
| Permanent pacemaker/ implantable cardioverter defibrillator | 42 (23%) | 22 (18%) | 0.3 |
∗ Defined as any coronary artery stenosis ≥50%, prior PCI or CABG.
Average SPAP was significantly higher in moderate/severe PH group (61 ± 12 mm Hg) compared with no/mild PH group (36 ± 9 mm Hg, p <0.001). This finding was corroborated also by pre-TAVR right-sided cardiac catheterization with intermediate correlation to the echocardiographic assessment of SPAP (r = 0.46; Table 2 ). Baseline echocardiographic evaluation showed that the average left ventricular function of the patients is preserved with only 16% and 12% of the patients who have LVEF <30% in both groups ( Table 2 ). Conversely, patients with moderate/severe PH had higher rates of concomitant valve disease and right ventricular failure. Similarly, rates of moderate/severe mitral regurgitation and moderate/severe tricuspid regurgitation were significantly higher and nearly twice as frequent as right ventricular dysfunction ( Table 2 ).
| Variable | Pulmonary hypertension | p Value | |
|---|---|---|---|
| No/mild (n=172) | Moderate /severe (n=243) | ||
| Echocardiogrpahy | |||
| Left ventricular ejection fraction (%±SD) | 53±17 | 53±14 | 0.9 |
| Left ventricular ejection fraction <30% | 38 (16%) | 21 (12%) | 0.3 |
| Aortic valve area (cm 2 ±SD) | 0.65±0.1 | 0.65±0.1 | 0.5 |
| Mean gradient (mmHg±SD) | 48±13 | 48±13 | 0.9 |
| Peak velocity | 4.4±0.6 | 4.4±0.6 | 0.7 |
| Septal thickness (cm±SD) | 1.3±0.2 | 1.3±0.2 | 0.6 |
| Posterior wall thickness (cm±SD) | 1.2±0.2 | 1.2±0.2 | 0.5 |
| Left ventricular end systolic diameter (cm±SD) | 3.1±0.9 | 3.2±0.9 | 0.2 |
| Left ventricular end diastolic diameter (cm±SD) | 4.4±0.8 | 4.4±0.8 | 1.0 |
| Moderate or severe mitral regurgitation | 19 (8.6%) | 29 (18.4%) | 0.007 |
| Moderate or severe tricuspid regurgitation | 13 (5.9%) | 38 (23%) | <0.001 |
| Moderate or severe RV dysfunction ∗ | 21 (19.8%) | 29 (35.3%) | 0.02 |
| Moderate or severe RV dilatation | 6 (2.6%) | 12 (7.3%) | 0.047 |
| Left atrial diameter (cm±SD) | 4.5±0.7 | 4.7±0.8 | 0.04 |
| Systolic pulmonary artery pressure (mmHg±SD) | 36±9 | 61±12 | <0.001 |
| Right sided heart catheterization † | |||
| Right atrial pressure; mean (mmHg±SD) | 8.9±7.9 | 8.9±5.5 | 1 |
| Pulmonary artery pressure (mmHg±SD) | <0.01 | ||
| Systolic | 44.9±13 | 57±16 | |
| Diastolic | 18.9±6 | 22.8±7 | |
| Mean | 28±8 | 34±9 | |
| Pulmonary capillary wedge pressure; mean (mmHg±SD) | 19±8 | 21±8 | 0.4 |
| Cardiac output (L/min±SD) | 4.2±1.2 | 4.6±1.5 | 0.3 |
| Cardiac index (L/min/m 2 ±SD) | 2.5±0.7 | 2.4±0.8 | 0.9 |
The procedural characteristics did not differ significantly between patients with no/mild and moderate/severe PH ( Table 3 ). There was a large majority for the transfemoral access and for the use of the SAPIEN transcatheter heart valve. Nearly 2/3 of the patients underwent the procedure with conscious sedation, thus avoiding the need for mechanical ventilation. Valve Academic Research Consortium inhospital complications were mostly comparable between the groups; however, acute kidney injury rates tended to be higher (nearly double) in patients with moderate/severe PH compared with patients with no/mild PH, although mostly driven by the grade 1 acute kidney injury rates. Interestingly, inhospital infection rates, typically respiratory, were higher in moderate/severe patients with PH. Finally, moderate/severe patients with PH had longer stay in intensive care; however, this did not translate to a longer hospitalization period ( Table 4 ).
| Variable | Pulmonary hypertension | p Value | |
|---|---|---|---|
| No/mild (n=172) | Moderate /severe (n=243) | ||
| Approach | |||
| Transfemoral | 179 (75%) | 134 (78%) | 0.3 |
| Transapical | 61 (25%) | 37 (22%) | |
| Valve type | |||
| SAPIEN | 167 (67%) | 110 (65%) | 0.6 |
| SAPIEN XT | 41 (17%) | 31 (18%) | |
| CoreValve | 33 (13%) | 24 (14%) | |
| Valve size (mm) | |||
| 23 | 129 (52%) | 88 (52%) | 0.8 |
| 26 | 90 (36%) | 66 (39%) | |
| 29 | 23 (9%) | 12 (7%) | |
| 31 | 6 (2.4%) | 4 (2.4%) | |
| Sedation | |||
| Conscious sedation | 154 (63%) | 112 (65%) | 0.7 |
| General anesthesia | 89 (37%) | 61 (35%) | |
| Fluoroscopy time (min. ± SD) | 22±21 | 19±10 | 0.1 |
| Contrast volume (ml ± SD) | 123±79 | 118±60 | 0.5 |
| Successful valve delivery | 227 (98%) | 159 (98%) | 0.7 |
| Complications | |||
| Tamponade | 1 (0.4%) | 3 (1.7%) | 0.3 |
| Balloon post dilatation | 19 (7.8%) | 8 (4.7%) | 0.2 |
| Need for second valve | 4 (1.8%) | 1 (0.7%) | 0.7 |
| Variable | Pulmonary hypertension | p Value | |
|---|---|---|---|
| No/mild (n=172) | Moderate /severe (n=243) | ||
| Major vascular complications | 23 (9.5%) | 16 (9.4%) | 0.9 |
| Life threatening bleed | 19 (7.9%) | 18 (10.6%) | 0.3 |
| Myocardial infarction | 1 (0.4%) | 1 (0.6%) | 1 |
| Stroke | |||
| Ischemic stroke | 12 (5%) | 10 (5.8%) | 0.7 |
| Hemorrhagic stroke | 0 | 0 | |
| Acute kidney injury | |||
| Any | 22 (10.1%) | 26 (17.2%) | 0.5 |
| Stage 1 | 15 (6.9%) | 18 (11.9%) | 0.09 |
| Stage 2 | 2 (0.9%) | 2 (1.3%) | 1 |
| Stage 3 | 5 (2.3%) | 6 (4%) | 0.4 |
| Mechanical ventilation post-procedure | 44 (18.2%) | 34 (20%) | 0.6 |
| Heart failure | 43 (17.9%) | 40 (23.4%) | 0.2 |
| New pacemaker | 14 (5.9%) | 12 (7%) | 0.6 |
| Any infection | 24 (10%) | 29 (17.1%) | 0.04 |
| Intensive care unit stay (days ± SD) | 2.7±3.7 | 3.9±6.3 | 0.03 |
| Post-procedure length of stay (days ± SD) | 6.3±7.4 | 7.8±8 | 0.5 |
| Death | 17 (7%) | 23 (13.5%) | 0.03 |
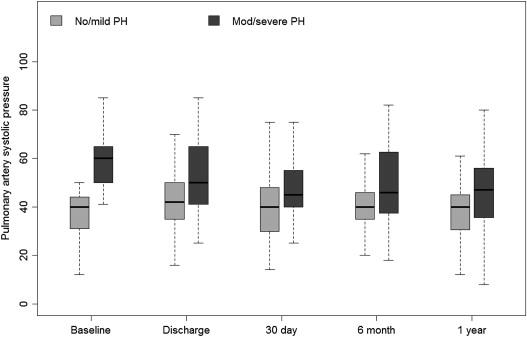
As demonstrated in Figure 2 , the elevated SPAP in the moderate/severe PH group tended to decrease after TAVR procedure. Interestingly, the decrease in SPAP occurred early, during the index hospitalization, and was maintained up to 1-year follow-up. However, SPAP values remained higher than the SPAP values among the patients in the no/mild PH group throughout follow-up and did not return at any stage to normal values ( Figure 2 ). Subgroup analysis of the moderate/severe PH group (n = 243) according to whether SPAP decreased early after procedure (defined as a decrease of ≥10 mm Hg) showed that although there is an early decrease in SPAP after procedure, the majority (n = 180, 74%) of the patients do not experience a significant improvement (i.e., ≥10 mm Hg). Furthermore, outcome was not different for the patients who experienced decrease in SPAP compared with those who did not with 7.4% versus 8% for 30-day mortality (p = 1) and 22.2% versus 28% for 1-year mortality (p = 0.56), respectively.