Present Concepts in the Molecular Biology of Lung Cancer
David H. Harpole Jr.
Mark Onaitis
Over the past decade, the study of cancer has increasingly focused upon molecular causes. This chapter offers a brief overview of molecular derangements in non-small-cell lung cancer and an introduction to two new and exciting approaches to the study of this disease: mouse models of cancer and genomics. These recent developments hold promise for both molecularly targeted therapeutics as well as individualized cancer treatment.
Oncogenes
Oncogenes are genes that lead to increased proliferation when overexpressed. Because they are activating mutations, mutation of overexpression of only one of two normal protooncogenes per cell is required for transformation. Oncogenes may lead to increased proliferation through a variety of mechanisms, including augmented growth rate, increased mitotic rate, and decreased rate of apoptosis.
Ras
The ras family of proteins consists of three members—H-ras, N-ras, and K-ras–the last of which is implicated in 30% to 40% of human lung adenocarcinoma.44 Wild-type ras is a membrane-associated G protein that serves as a link between tyrosine kinase receptors at the membrane and cytoplasmic second messenger molecules. These second messenger molecules participate in a proliferative signaling cascade that activates mitogen-activated protein kinase (MAPK), resulting in increased proliferation. This proliferation is caused by mutations in K-ras codons 12, 13, and 61, all of which ablate K-ras’s GTPase activity and constitutively activate it.
In both individual studies and a meta-analysis, the presence of mutated K-ras in resected lung cancer portends a poor prognosis.34,48,51 Although the presence of these mutations may identify aggressive tumors, a portion of the prognostic impact may be explained by poor response to adjuvant chemotherapy. In the JBR.10 trial, the subgroup of patients with ras mutations did not experience a survival advantage.54 Because localization of ras to the membrane is dependent upon farnesylation, farnesyltransferase inhibitors may be more effective in patients with a ras mutation.
In addition to mutation of ras, control of normal ras protein levels may be important in lung cancer tumorigenesis. Recently, ras was found to be an important target of the let-7 microRNA family. MicroRNAs are 22-nucleotide RNA molecules that regulate gene expression negatively by silencing messenger RNAs or targeting microRNAs for destruction. Lung tumors exhibit lower levels of let-7 than normal lung,24 and low expression levels of let-7 have been demonstrated as predictive of poor survival.56,57
Epidermal Growth Factor Receptor (EGFR)
Unlike ras, EGFR mutations most commonly occur in lung tumors of nonsmokers. They virtually never occur in the presence of a ras mutation and are most commonly present in adenocarcinomas of East Asian females.46,47 Along with Her2, Her3, and Her4, EGFR receptors make up one of four types of ErbB receptors. Ligands of EGFR are varied, including both epidermal growth factor and transforming growth factor alpha.17 Ligand binding of the receptor leads to dimerization, phosphorylation, and activation of signaling pathways, leading to proliferation.
Because of the availability of the EGFR-specific tyrosine kinase inhibitors, EGFR mutation has received much recent scrutiny. The majority of these mutations occur as either in-frame deletions of exon 19 or missense mutation in exon 21.20,30,33,38,39,46,53 Epithelial cells with mutant EGFR probably become physiologically dependent on the gene’s continued activity for maintenance of the transformed phenotypes.14 As Figure 106-1 reveals, patients with tumors expressing EGFR and Her-2-neu may have poorer postoperative survival than those with one or neither positive.
Tumor Suppressors
A tumor suppressor is a gene whose absence allows neoplasia to progress. Because even a small amount of wild-type protein may suppress transformation, homozygous deletion of a tumor
suppressor is thought necessary for oncogenesis. When a patient is heterozygous for a gene, the term loss of heterozygosity (LOH) is used to describe deletion or inactivation of the wild-type allele (Fig 106-2).
suppressor is thought necessary for oncogenesis. When a patient is heterozygous for a gene, the term loss of heterozygosity (LOH) is used to describe deletion or inactivation of the wild-type allele (Fig 106-2).
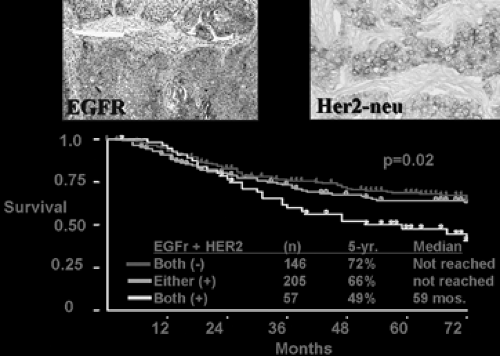 Figure 106-1. Unpublished data from the Harpole laboratory demonstrating diminished survival in stage I patients with tumors staining positive for both EGFR and Her-2-neu. |
p53
Perhaps the best-studied tumor suppressor gene is p53, which encodes a transcription factor with diverse biological functions, including the mediation of cell cycle arrest and cell death after DNA damage. The loss of wild-type (normal) p53 activity is the most common genetic abnormality in human cancer and can occur through allelic deletions, point mutations, or both that result in amino acid substitutions that alter protein function. Abnormalities in p53 are also the most frequent genetic alterations in lung cancer, occurring in 90% of small-cell lung cancers (SCLCs) and up to 60% of non-small-cell lung cancers (NSCLCs). These alterations primarily involve inactivating point mutations, but deletions, rearrangements, and splice mutations have also been described. As with ras mutations, p53 mutations are more common in smokers. Denissenko and coworkers10, reported that benzo[a]pyrene, a major carcinogen in tobacco smoke, preferentially forms adducts at the guanine positions in p53 that are most frequently mutated in human lung cancer. In addition, in 2000, Ahrendt and associates2 found that p53 mutations occur much more frequently in lung cancers from patients with a history of significant alcohol and tobacco use.23
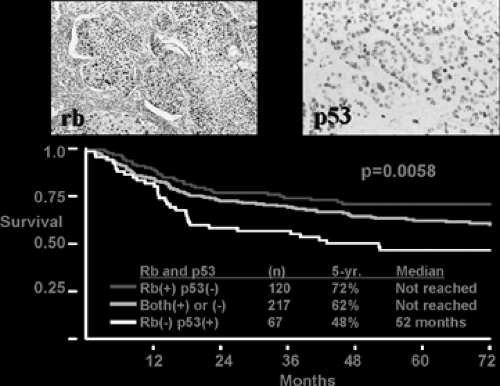 Figure 106-2. Unpublished data from the Harpole laboratory revealing diminished survival in stage I patients with Rb deficiency and p53 overexpression. |
The prognostic significance of p53 abnormalities in lung cancer remains controversial. Since the half-life of mutant p53 protein is usually significantly longer than that of the wild-type protein, the detection of p53 by immunohistochemistry is considered a marker for p53 mutation, although Carbone and associates7 in 1994 found that the concordance between positive p53 immunostaining and the presence of a p53 mutation was only 67%.24 Numerous studies have reported that p53 mutation or overexpression is predictive of poor prognosis, whereas others have found no association between p53 mutations and survival in either NSCLC or SCLC. Two recent meta-analyses concluded that the presence of p53 mutation or overexpression correlates with a poorer prognosis in patients with NSCLC.34,49 Data from the Harpole lab is in agreement with this (see Fig. 106-2).
p16
Cytogenetic studies have identified chromosomal region 9p21 as one of the most commonly affected loci in human cancers, including lung cancer. The p16 (INK4a, CDKN2A) gene, located at 9p21, has been characterized as a tumor suppressor gene based on the high frequency of p16 mutations and deletions present in a wide variety of human tumors and the presence of germline mutations in families with hereditary melanoma. The p16 protein is one of a family of cdk inhibitors that regulate progression through the cell cycle by inactivating cyclin-dependent kinase 4 (cdk4) or cdk6, thereby inhibiting the phosphorylation of retinoblastoma 1 (Rb1) by cyclin D:cdk4/6 complexes. Unphosphorylated Rb1 binds E2F, with resultant cell cycle arrest at the G1/S boundary. Therefore the loss of p16 function or the overexpression of cyclin D1 would have the same net effect as the loss of Rb1 function: unregulated passage through the G1/S checkpoint with resultant neoplastic proliferation. At least one of these three abnormalities—loss of Rb1, loss of p16, or overexpression of cyclin D1—occurs in most if not all human cancers, suggesting that dysregulation of the Rb–cyclin D–cdk4/6–p16 pathway controlling the G1/S transition may be a necessary step during malignant transformation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


