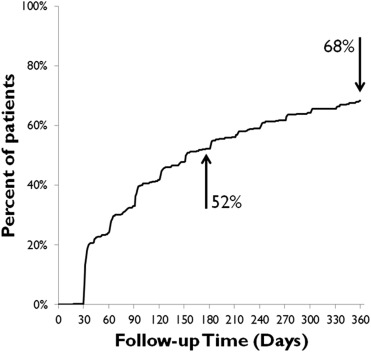
Premature clopidogrel discontinuation is an important cause of stent thrombosis, myocardial infarction, and death after drug-eluting stent (DES) placement. Previous studies of clopidogrel nonadherence after DES placement have had short follow-up and have relied on self-reported adherence. All patients who underwent DES placement from January 2008 to December 2011 at a single safety-net hospital and received medications through the county-subsidized health plan were considered for inclusion; those with <1-year follow-up were excluded. We retrospectively collected 1-year refill data from a large, closed pharmacy system. Our primary outcome was time to failure to obtain a clopidogrel refill, allowing a 5-day grace period. Our study cohort (n = 369) was 34% female, 39% Hispanic, 26% white, and 26% African-American; 26% identified Spanish as their primary language. The time to failure to obtain a clopidogrel refill was 153 days. Cumulative failure to obtain at least 1 refill was 23% for the first refill, increasing to 52% at 6 months and 68% at 1 year. Examining the proportion of days covered (PDC), 21% of patients had 100% coverage, whereas 34% had PDC <80%. There were no independent predictors of nonadherence (PDC <80%). In conclusion, we identified a high rate of clopidogrel nonadherence in a multiethnic urban poor patient population where clopidogrel was provided at discharge and at nominal cost thereafter. In this cohort, prediction models of nonadherence performed poorly. Novel strategies are needed to address this important problem.
No previous study has examined clopidogrel nonadherence after drug-eluting stent (DES) placement in the urban poor, who may face unique challenges in adhering to a treatment plan. Moreover, demographic and clinical factors associated with low clopidogrel adherence have not been described in this patient population.
Methods
We conducted a retrospective cohort study of clopidogrel adherence for 1-year after DES placement, using pharmacy refill data from a closed pharmacy system (county health system subsidized care). During the study period, Parkland Health and Hospital System (PHHS) (Dallas, Texas), through the Parkland Health Plus (PHP) program, provided taxpayer-subsidized assistance for uninsured low-income Dallas County residents who qualified based on income and household size. PHP patients may receive a multitude of subsidies, allowing office visits, prescriptions through a closed formulary, emergency care, and hospitalization at nominal cost. This study was conducted in the PHHS, and the protocol was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and by PHHS. Clopidogrel was provided by Parkland pharmacy to PHP patients at a nominal cost.
Records of all patients undergoing cardiac catheterization from January 2008 to December 2011 were screened for eligibility, and a comprehensive chart review was performed (HK, RS, MW, and AE). All indications for percutaneous coronary intervention (PCI) were considered eligible. Inclusion criteria included (1) PHP enrolled, (2) coronary DES implantation, (3) clopidogrel prescription at discharge, and (4) ≥1 year of post-PCI follow-up. Exclusion criteria included (1) switching to an alternative P2Y 12 platelet inhibitor during follow-up, (2) obtaining alternative health insurance during follow-up, (3) discontinuation of clopidogrel by a physician provider (e.g., due to bleeding or need for surgery), (4) having prescriptions sent to an outside pharmacy, and (5) cardiologist-adjudicated noncardiac death during follow-up.
Our primary outcome was time to failure (TTF) to obtain clopidogrel refill in the year after index PCI. Because clopidogrel has an antiplatelet effect persisting after medication discontinuation, we allowed a 5-day grace period. We also examined clopidogrel adherence by calculating the proportion of days covered (PDC) during the 1-year follow-up period; PDC ranges from 0% (no adherence) to 100% (perfect adherence). Adherence was defined based on the traditional dichotomous definition of adherence (PDC cut-off of ≥ and <80%). Finally, we calculated the proportion of patients who successfully obtained a refill within 5 days after their discharge prescription ended. In cases of multiple PCIs during the study period, only refill data after the last PCI were included.
Continuous variables are expressed as mean ± SD, and categorical variables are expressed as counts and percentages. Baseline characteristics of patients were compared using the Fisher’s extract test for categorical variables and t test for continuous variables.
Univariate logistic regression was used to identify predictors of nonadherence (PDC <80%), and variables with statistical significance were included in a multivariate logistic regression model; candidate variables included ejection fraction <40%, clinical history of congestive heart failure, history of coronary artery disease (CAD), smoking history, and preferred language. All analyses were performed with SAS software (version 9.2; SAS Institute, Cary, North Carolina). p Values <0.05 were considered statistically significant, and all tests of statistical significance were 2-tailed.
Results
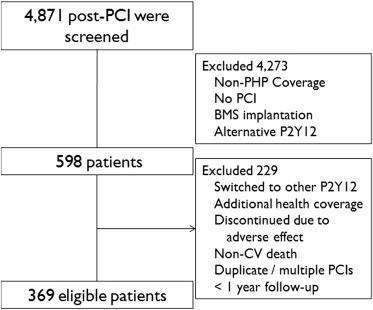
Of 4,871 patients screened, 598 patients met inclusion criteria, and 229 were excluded ( Figure 1 ). The study cohort was predominantly male (66%), and the mean age was 55 ± 9 years ( Table 1 ). The most common reason for PCI was stable angina (53%), followed by non–ST elevation myocardial infarction (MI) or unstable angina (39%) and ST elevation MI (8%). The proportion of patients self-identified as Hispanic was 39%, white 26%, and black 26%. The proportion of the study population who spoke primarily Spanish was 26%. More than half the patients had a previously established diagnosis of CAD (56%), 40% had undergone previous PCI, and 32% had been taking clopidogrel before the index PCI.
| Characteristics | Overall n=369 | PDC ≥ 80% n=244 | PDC < 80% n=125 | P value |
|---|---|---|---|---|
| Age (years) | 55 (35-86) | 55.0 | 54.9 | 0.21 |
| Female | 124 (33.6%) | 34.2% | 32.6% | 0.82 |
| Race or ethnic group | ||||
| White | 97 (26.3%) | 27.9% | 23.3% | 0.39 |
| Hispanic | 142 (38.5%) | 40.8% | 34.1% | 0.22 |
| Black | 95 (25.7%) | 21.7% | 34.1% | 0.013 |
| Other | 35 (9.5%) | 9.6% | 9.3% | 1.0 |
| Preferred Language | ||||
| English | 264 (71.5%) | 67.9% | 78.3% | 0.04 |
| Spanish | 96 (26.0%) | 30.0% | 18.6% | 0.018 |
| Other | 9 (2.4%) | 2.1% | 3.1% | 0.73 |
| Indication for PCI | ||||
| STEMI | 29 (7.9%) | 8.3% | 7.0% | 0.69 |
| NSTEMI/UA | 145 (39.3%) | 39.6% | 38.8% | 0.91 |
| Stable Angina | 194 (52.6%) | 51.7% | 54.3% | 0.66 |
| Comorbidities | ||||
| Diabetes Mellitus | 208 (56.4%) | 55.4% | 58.1% | 0.66 |
| COPD | 57 (15.4%) | 14.6% | 17.1% | 0.55 |
| Depression | 98 (26.6%) | 14.2% | 20.2% | 0.14 |
| GI Bleed | 12 (3.3%) | 2.5% | 4.7% | 0.36 |
| Active Smoker | 108 (29.3%) | 25.8% | 35.7% | 0.055 |
| Hospitalization | 1.27% | 1.2% | 1.4% | 0.46 |
| Cardiovascular disease history | ||||
| Prior CAD | 208 (56.4%) | 52.1% | 64.3% | 0.028 |
| Prior PCI | 148 (40.1%) | 38.8% | 42.6% | 0.50 |
| Clinical CHF | 98 (26.6%) | 22.9% | 33.3% | 0.036 |
| LV Ejection Fraction < 40% | 54 (14.6%) | 10.4% | 22.5% | 0.003 |
| Medications | ||||
| Number of Medications | 10.4 | 10.3 | 10.6 | 0.53 |
| On Warfarin | 6 (1.6%) | 0.8% | 3.1% | 0.19 |
| Prior Clopidogrel | 118 (32.0%) | 31.7% | 32.6% | 0.91 |
| Homeless | 3 (0.8%) | 0.4% | 0.16% | 0.27 |
The median TTF to obtain a clopidogrel refill was 153 days. Cumulative failure to obtain at least 1 refill (allowing a 5-day grace period) was 23% for the first refill, increasing to 52% at 6 months and 68% at 1 year ( Figure 2 ). Examining the PDC, by 1-year follow-up, 21% of patients had 100% PDC and 34% had PDC <80% ( Figure 3 ). Mean PDC was 81% at the end of the study period.