The objective was to examine real-world treatment patterns of lipid-lowering therapies and their possible associated intolerance and/or ineffectiveness in patients with high cardiovascular disease (CVD) risk initiating statins and/or ezetimibe. Patients aged ≥18 years who initiated statins and/or ezetimibe from January 01, 2007, to June 30, 2011, were retrospectively identified from the IMS LifeLink PharMetrics Plus commercial claims database. Patients were further classified into 2 cohorts: (1) history of cardiovascular event (CVE) and (2) history of coronary heart disease risk equivalent (CHD RE). Patients had continuous health plan enrollment ≥1 year pre- and post-index date (statin and/or ezetimibe initiation date). Primary outcomes were index statin intensity, treatment modifications, possible associated statin/nonstatin intolerance and/or ineffectiveness issues (based on treatment modification), and time-to-treatment modifications. Analyses for each cohort were stratified by age group (<65 and ≥65 years). A total of 41,934 (history of CVE) and 170,344 patients (history of CHD RE) were included. On the index date, 8.8% to 25.1% of patients were initiated on high-intensity statin. Among patients aged <65, 79.2% and 48.8% of those with history of CVE and 78.6% and 47.3% of those with a history of CHD RE had ≥1 and 2 treatment modifications, respectively. Among all patients, 24.6% to 25.6% had possible statin intolerance and/or ineffectiveness issues after accounting for second treatment modification (if any). In conclusion, in patients with high CVD risk, index statin treatment modifications that imply possible statin intolerance and/or ineffectiveness were frequent; low use of high-intensity statins indicates unmet need in the management of hyperlipidemia and possible remaining unaccounted CVD residual risk.
Despite the guidelines recommendation and efficacy of statins, not all patients, based on their cardiovascular risk, can adequately control their low-density lipoprotein cholesterol (LDL-C) on the starting statin dose; therefore, lipid-lowering treatment (LLT) regimens may be modified. These modifications may include dose escalation, switches to different LLT agents, or augmentation with other LLTs. Some statin treatment modifications may potentially be indicative of index statin intolerance and/or ineffectiveness. There are no standardized definitions or US Food and Drug Administration diagnostic criteria for statin intolerance. Typically, it is defined as the inability to use statins because of significant symptoms (muscle myalgia and gastrointestinal side effects) or elevated creatine kinase levels. Several national and international medical societies recommend rechallenge of statin treatments and/or switching to other LLTs as possible management options for statin-associated muscle symptoms. The American Heart Association (AHA)/American College of Cardiology (ACC) 2013 guidelines indicate that therapy ineffectiveness is a less-than-anticipated therapeutic response to statin therapy in which upward titration of statin dose or combination therapy is considered. Statin treatment intolerance and/or ineffectiveness in patients with high risk could potentially result in residual elevated risk for costly atherosclerotic cardiovascular disease (CVD) events. This study examined LLT modifications in patients with high CVD risk initiating statins and/or ezetimibe. This study also investigated how such modifications may be associated with possible treatment intolerance and/or ineffectiveness.
Methods
This was a retrospective cohort analysis using the IMS LifeLink PharMetrics Plus commercial claims database from January 1, 2006, to June 30, 2012. This nationally representative, longitudinal database comprised managed care health plan information throughout the United States, with adjudicated claims of >150 million enrollees since 2006.
Eligible patients aged ≥18 years with >1 outpatient pharmacy claims for statins and/or ezetimibe from January 1, 2007, to June 30, 2011, from the IMS LifeLink PharMetrics Plus Dataset were identified. The initiation date of the first statin and/or ezetimibe was designated as the index date. The second outpatient pharmacy claim for statins and/or ezetimibe was required to be ≤6 months apart. Two prescriptions were required as part of the inclusion criteria to eliminate patients who received only 1 prescription and never refilled and, hence, would not have had a possibility for a LLT modification since initiation of therapy. All patients were required to have continuous health plan enrollment for ≥12 months pre-index date (baseline period) and 12 months post-index date. The follow-up period for treatment patterns varied in length from (and including) the index date to the end of continuous health plan eligibility or the end of the study period (June 30, 2012), whichever occurred first. During this study period, the National Cholesterol Education Program’s Adult Treatment Panel III guidelines were the current treatment guidelines and the AHA/ACC 2013 guidelines were not yet adopted.
Patients with ≥1 outpatient pharmacy claim for statin and/or ezetimibe in the 12 months before the first index statin and/or ezetimibe claim and those with medical claims indicating pregnancy or delivery ( International Classification of Diseases, Ninth Revision, Clinical Modification [ ICD-9-CM ] codes in Appendix A ) at any time during the baseline or follow-up period were excluded; patients with >2 different index statins on the index date were excluded.
Patients were stratified into the following 2 high CVD-risk cohorts:
- 1)
History of cardiovascular event (CVE): Patients with a history of CVEs, defined as ≥1 nondiagnostic medical claim (inpatient or outpatient) with a diagnosis or procedure code for myocardial infarction, unstable angina, ischemic stroke, coronary artery bypass graft surgery, percutaneous coronary intervention, or transient ischemic attack ( ICD-9-CM codes in Appendix A ) during the baseline period; and
- 2)
History of coronary heart disease risk equivalent (CHD RE): Patients with a history of CHD RE, defined as ≥1 nondiagnostic medical claim (inpatient or outpatient) with a diagnosis or procedure code for peripheral artery disease, abdominal aortic aneurysm, ischemic heart disease, stable angina, or type 2 diabetes mellitus ( ICD-9-CM codes in Appendix A ), but no history of CVE during the baseline period.
Demographic and clinical characteristics of patients in each cohort were examined. Initial statin intensity (low, moderate, and high) was also recorded and stratified by index year for each cohort. Using average daily dose, definitions of different statin intensities were adapted from the 2013 ACC/AHA guidelines ( Appendix B ). The average daily dose was defined as the strength of the statin multiplied by the dose quantity and divided by the total days of supply.
Treatment modification (none, first, and second) and time-to-treatment modification (days) during the follow-up period were analyzed. Treatment modifications are defined in Table 1 . Treatment modifications included dose escalation, dose reduction, augmentation, subtraction for patients receiving combination therapy, reinitiation of index therapy, switching, and permanent LLT discontinuation. Using treatment modifications observed in the claims data, statin dose reduction, temporary discontinuation followed by reinitiation of the same statin, and subtracting the statin from a combination therapy were considered as a signal for possible LLT intolerance concerns. LLT ineffectiveness, used in this study, was defined in a similar way as the AHA/ACC 2013 guidelines (i.e., less-than-anticipated therapeutic response) and treatment modifications, such as dose escalation and augmentation with a non-statin LLT, were considered as possible LLT ineffectiveness. To increase the likelihood that the treatment modifications were associated with treatment intolerance and/or ineffectiveness, we categorized and accounted for both the observed first and second treatment modifications (if any) into possible LLT intolerance and/or ineffectiveness issues (defined in Appendix C ). For example, treatment modifications of switching to a different statin or discontinuation of statin were categorized as possible statin intolerance and/or ineffectiveness. Patients with no treatment modification, or those who had a first treatment modification but no second treatment modification, were classified into low-/moderate-intensity statin and/or ezetimibe or high-intensity statin treatment with/without ezetimibe treatment depending on their statin intensity and/or ezetimibe treatment.
| Treatment Modification | Definition |
|---|---|
| Dose escalation | Continuation of index treatment with dose escalation of ≥25% between consecutive claims of statin |
| Dose reduction | Continuation of index treatment with dose reduction of ≥25% between consecutive claims of statin |
| Augmentation | Continuation of index treatment with addition of a new non-statin medication (or addition of a statin medication for those with ezetimibe as index treatment) |
| Subtraction | Moved from combination treatment to a subset of combination treatment/monotherapy within end of days’ supply plus 60-day grace period |
| Switch to non-index statin | Initiation of non-index statin post-index treatment date (Termination of index statin treatment was assumed if days’ supply overlapped) |
| Switch to non-statin | Initiation of non-statin post-index treatment date |
| Reinitiation | Discontinuation of all components of index treatment and reinitiate index treatment after end of days’ supply plus 60-day grace period |
| Permanent discontinuation | Discontinuation of all components of index treatment through end of follow-up period |
All measures, including patients’ demographic and clinical characteristics, between the 2 cohorts were reported. Analyses for each cohort were stratified by age groups (<65 and ≥65 years) to investigate the impact (if any) of Medicare eligibility.
Results
After applying inclusion and exclusion criteria, 41,934 patients had a history of CVE and 170,344 had a history of CHD RE during the baseline period ( Appendix D ).
The average ages of patients with a history of CVE and history of CHD RE were 58 and 57 years, respectively ( Table 2 ). The mean (±SD) Deyo-Charlson Comorbidity Index score for patients in the history of CVE cohort was 1.9 (±1.7) and was 1.5 (±1.3) for patients in the history of CHD RE cohort ( Table 2 ). Study results for those aged <65 years are reported subsequently; similar patterns were also observed for patients aged ≥65 years.
| Variable | History of CVE (N=41,934) | History of CHD RE (N=170,344) |
|---|---|---|
| Age (Mean and SD) (Years) | 57.6 (10.2) | 56.6 (10.8) |
| Age by Medicare Category (Years) | ||
| <65 | 34674 (82.7) | 140372 (82.4) |
| ≥65 | 7260 (17.3) | 29972 (17.6) |
| Gender | ||
| Male | 28113 (67.0%) | 97559 (57.3%) |
| Geographic Distribution | ||
| Northeast | 12780 (30.5%) | 54310 (31.9%) |
| North Central | 9003 (21.5%) | 42990 (25.2%) |
| West | 16987 (40.5%) | 53242 (31.3%) |
| South | 3164 (7.5%) | 19802 (11.6%) |
| Payer Type | ||
| Health Maintenance Organizations | 5764 (13.7%) | 29489 (17.3%) |
| Indemnity | 2547 (6.1%) | 11019 (6.5%) |
| Preferred Provider Organizations | 30014 (71.6%) | 106923 (62.8%) |
| Point of Service | 3441 (8.2%) | 22027 (12.9%) |
| Consumer-directed Health Care | 33 (0.1%) | 585 (0.3%) |
| Unknown/Missing | 135 (0.3) | 301 (0.2%) |
| Baseline Deyo-Charlson Comorbidity Index Categories | ||
| Mean (SD) | 1.9 (1.7%) | 1.5 (1.3%) |
| 0 | 5714 (13.6%) | 23429 (13.8%) |
| 1 | 16813 (40.1%) | 96934 (56.9%) |
| 2 | 9083 (21.7%) | 21149 (12.4%) |
| ≥3 | 10324 (24.6%) | 28832 (16.9%) |
| Baseline Comorbidities | ||
| Hyperlipidemia | 30309 (72.3%) | 133091 (78.1%) |
| Hypertension | 30017 (71.6%) | 116933 (68.6%) |
| Arrhythmias | 11559 (27.6%) | 14118 (8.3%) |
| Diabetes (Type 1 or 2) | 9907 (23.6%) | 127234 (74.7%) |
| Chronic Respiratory Disease | 7624 (18.2%) | 20685 (12.1%) |
| Carotid Artery Disease | 5707 (13.6%) | 4612 (2.7%) |
| Obesity | 5355 (12.8%) | 17629 (10.3%) |
| Congestive Heart Failure | 5096 (12.2%) | 5818 (3.4%) |
| Peripheral Arterial Disease | 4522 (10.8%) | 15686 (9.2%) |
| Myalgia/muscle weakness | 3189 (7.6%) | 8475 (5.0%) |
| Cancer (excluding non-melanoma skin cancer) | 2515 (6.0%) | 10065 (5.9%) |
| Liver Disease | 901 (2.1%) | 4424 (2.6%) |
| Hemorrhagic Stroke | 697 (1.7%) | 221 (0.1%) |
| Metabolic Syndrome | 512 (1.2%) | 3025 (1.8%) |
| End-stage Renal Disease | 403 (1.0%) | 1115 (0.7%) |
| Baseline Concomitant Medications | ||
| Anti-hypertensive Medications | 23156 (55.2%) | 102604 (60.2%) |
| Oral Antidiabetic Medications | 4717 (11.2%) | 71416 (41.9%) |
| Insulin | 1639 (3.9%) | 16143 (9.5%) |
In both cohorts, from 2007 to 2011, in patients aged <65 years, 6.7% to 20.9% initiated a low-intensity statin, 66.6% to 77.8% initiated a moderate-intensity statin, and 8.8% to 25.1% initiated a high-intensity statin.
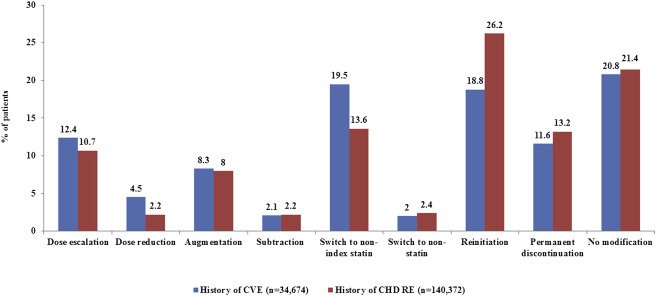
Figure 1 shows the percentage of patients with an initial treatment modification or no modification for both cohorts, among patients aged <65 years. Of those, 79.2% and 78.6% in the history of CVE and history of CHD RE cohorts, respectively, had at least 1 treatment modification. In the history of CVE cohort, patients aged ≥65 years had the same top 2 most common treatment modifications as those aged <65 years. In the history of CHD RE cohort, the top 3 most common treatment modifications were the same for patients aged <65 and ≥65 years. Treatment trends among patients aged ≥65 years are available in Appendix E .