Fig. 13.1
Chest tube collecting system—contemporary chest tube collecting systems reproduce the traditional “three-bottle system”. The first compartment collects fluid drained from the pleural space. Air then passes to the second compartment, where a column of water acts as a one-way seal between the collection canister and the atmosphere. Air leaks can be visualized at the level of the second compartment. The third compartment allows negative pressure to be applied to the chest tube. Source: UpToDate
Negative pressure applied to the chest tube system increases the rate of lung re-expansion following pneumothorax. However, once the lung is completely re-expanded, the chest tube can be placed to underwater seal.
Traditionally, patients with a chest tube in situ have undergone daily chest radiography. There is no data to support this practice, and it is associated with significant cost and unnecessary discomfort to the patient [11]. Multiple studies have demonstrated that, following lung resection and with chest tube placement, daily chest radiographs in asymptomatic patients contribute minimally to patient management decisions [12]. As with any investigation, thought should be given to the indication for the test. Early after chest tube insertion, chest radiography allows visualization of lung re-expansion and drainage of hemothorax. A chest radiograph should also be ordered to investigate any respiratory symptoms which could indicate either pneumonia or a chest tube complication: shortness of breath, tachypnea, increased pain, increased oxygen requirements or hypoxia. Finally, a chest radiograph should be performed whenever any modifications are performed on the chest tube, whether this be repositioning or modification of the collection system (change in suction, tipping over of the collection canister).
In the case of persistent pleural fluid on chest radiography 48 h following chest tube insertion, CT should be performed to assess the patient for significant retained hemothorax [2]. If hemothorax persists despite previous tube thoracostomy, additional drainage is required. Persistent hemothorax after primary chest tube insertion is associated with a 33 % risk of empyema [13]. Although it might appear reasonable to attempt drainage of retained hemothorax with a second chest tube, this may not be optimal. In a small randomized controlled trial comparing surgical evacuation [video-assisted thoracoscopic surgery (VATS)] with the insertion of a second chest tube, patients undergoing VATS had shorter length of stay and shorter need for chest tube drainage [14]. Furthermore, almost half of the patients treated with a second chest tube required surgical intervention. Retained hemothorax should prompt expert consultation and should be considered for surgical evacuation within 3–7 days of admission [2].
Air leaks in the trauma population generally resolve within 48 h of injury. Patients who develop a persistent air leak following chest wall injury require further evaluation to rule out underlying injury to the lung. The EAST guidelines recommend VATS evaluation for an air leak that persists beyond 48 h after admission, though this recommendation is based only on moderate evidence [2]. Persistent air leak should prompt consultation with a thoracic surgeon.
Complications of Tube Thoracostomy
Complications associated with tube thoracostomy can occur during insertion, while the chest tube is in situ, and during chest tube removal. The most feared complication of chest tube insertion is injury to intrathoracic or intra-abdominal structures. Although injuries to the lung parenchyma and the intercostal bundle are most common, injuries to the heart, mediastinum, diaphragm, spleen, liver and even hollow viscus can occur. To avoid these (potentially fatal) injuries, landmarking for chest tube insertion must be meticulous, and care must be taken to enter the pleural cavity in a controlled fashion. Special caution should be taken in patients with previous thoracic surgery, difficult landmarking due to obesity and those at high risk of having pleural adhesions (history of tuberculosis, multiple previous lung infections).
While a chest tube is in place, the patient is at risk of complications related to the malfunction of the chest tube system, as well as infectious complications related to having a foreign body in the pleural cavity. Chest tubes can migrate, kink or become disconnected from the collecting system. Although any leak in the chest tube/collecting system can cause pneumothorax, a kink in the chest tube prevents air from escaping the thoracic cavity. Air accumulated in the thoracic cavity can cause tension pneumothorax, which can lead to severe respiratory and hemodynamic compromise. Kinking of the chest tube can occur iatrogenically if a clamp is applied to the chest tube and left in place; for this reason, chest tubes should never be left clamped, except in a highly monitored setting under very select circumstances.
Chest Tube Removal
The timing of chest tube removal depends largely on the initial indication for insertion. If the chest tube was inserted purely for pneumothorax and the lung has re-expanded completely, removing the chest tube after 24 h is reasonable. Recommendations regarding the appropriate timing of chest tube removal in the case of pleural collection vary widely; daily rates of drainage from 100 to 400 mL have been cited as an indication for chest tube removal [15]. In our practice, chest tubes are left in place until drainage is 100 mL or less per day.
If the chest tube is under negative pressure at the time when chest tube removal is considered, it should be placed to underwater seal for 6–8 h prior to removal [16]; a chest radiograph should be obtained after this time interval to ensure that the lung remains re-expanded. Chest tubes should be removed when the patient’s intrathoracic pressure is positive, in order to avoid re-accumulation of a pneumothorax.
Whether a chest radiograph needs to be obtained after chest tube removal is somewhat controversial. A systematic review of studies examining routine chest radiography in the context of cardiothoracic surgery concluded that routine post-chest tube removal X-rays are not beneficial and that patients requiring re-intervention (i.e. reinsertion of chest tube) have symptoms that would prompt imaging [17]. In the elective cardiothoracic surgery patient, however, the incidence of post-chest tube removal complications may be lower than in the trauma population, both because patients following cardiac surgery have a lower a priori risk of pneumothorax and because of the conditions under which the chest tube was originally inserted (in the operating room, with minimal tissue dissection). In addition, for many patients with chest wall trauma, chest tube removal is the last event that occurs prior to discharge to home; as such, discharging patients without chest radiography could put them at risk of deteriorating outside the hospital.
Analgesia Following Chest Wall Injury/Surgery
Meticulous analgesia following operative fixation of chest wall injuries is essential to ensuring optimal patient outcomes. As with patients whose injuries are being managed non-operatively, the optimal analgesic regiment for patients who have undergone surgery must be tailored to each individual patient. Among patients who have undergone thoracotomy for the purpose of chest wall fixation, strong consideration should be given to regional anaesthesia techniques (among patients who have no contraindications to epidural or paravertebral nerve blocks). Close collaboration with the anaesthesia team, as well as a multimodal approach to pain management, should be standard in this patient population.
Direct operative exposure provides additional opportunities to improve postoperative pain control. Specifically, intercostal nerve blocks can easily be administered under direct visualization in the operative room prior to chest wall closure. In addition, where expertise exists, paravertebral nerve blocks can also be administered intra-operatively.
A comprehensive approach to pain control is essential to avoiding adverse events. Adequacy of analgesia is closely related to the patient’s ability to mobilize and maintain normal respiratory function and pulmonary toilet. Pain prevents patients from coughing and taking deep breaths and leads to a drop in functional residual capacity (FRC). These changes are associated with atelectasis, inadequate clearance of pulmonary secretions and suboptimal ventilation and oxygenation. Inadequate analgesia is linked to increased incidence of pneumonia and increased mortality.
Analgesic options commonly used following chest wall trauma include systemic opioids, non-opioid analgesics and a variety of regional anaesthetic techniques. The latter category includes intercostal blocks, epidural anaesthesia (opioid, local anaesthetic or combined), paravertebral blocks and intrapleural injections. Each of these modalities is associated with advantages and disadvantages that must be tailored based on the patient’s underlying injuries, mobility, frailty and preference. Close collaboration with the anaesthesia team is essential to optimize outcomes among patients with chest wall injuries [18].
Opioids can be administered orally, intravenously on an intermittent basis, as a continuous infusion, or intravenously by means of patient controlled analgesia. Opioid analgesia is often most easily ordered and administered on a standard surgical ward; this approach may be adequate for patients with minimal injuries. However, opioids suppress respiratory drive and cough and may therefore exacerbate the negative respiratory effects associated with chest wall injuries. Furthermore, opioid analgesia used alone is often inadequate to suppress pain caused by severe chest wall injuries. Opioids are therefore best used in the context of multimodal analgesia, both to ensure adequate analgesia and to minimize the total dose received by the patient. In the absence of contraindications, acetaminophen and nonsteroidal anti-inflammatory drugs should routinely be used in conjunction with opioids.
Intercostal nerve blocks with a long-acting local anaesthetic are an attractive option in patients with localized pain due to a small number of closely spaced rib fractures. The procedure involves injection of local anaesthetic posterior to the mid-axillary line at the level of the intercostal bundle (inferior to the rib), blocking the lateral cutaneous and anterior branch of the intercostal nerve. Typically, a block needs to be administered up to one rib level above and one rib level below the injury. Intercostal nerve blocks in a patient with rib fracture at multiple levels are not optimal; in these patients, intercostal nerve block is time consuming, is painful for the patient and might require excessively high doses of local anaesthetic.
Epidural analgesia involves infusion of local anaesthetic, opioids or both into the epidural space at the level of the thoracic or lumbar spine. Epidural analgesia can provide pain relief superior to that provided by opioids. The EAST Practice Management Guidelines for analgesia in blunt chest trauma recommend epidural analgesia in the setting of multiple rib fractures, particularly in elderly patients with more than four rib fractures and in patients with flail chest [19]. These guidelines included data from both randomized and non-randomized studies. A recent meta-analysis of randomized controlled trials of epidural analgesia in the context of rib injuries found no reduction in mortality or length of stay [20]. A large number of patients are not candidates for epidural analgesia; epidural analgesia is contraindicated in patients with ongoing hemodynamic instability or hypovolemia, given that epidural analgesia can lead to further hypotension. Finally, the insertion of an epidural catheter can be technically difficult in a patient with significant pain and limited mobility and is not technically feasible in patients with significant spinal injuries or coagulopathy.
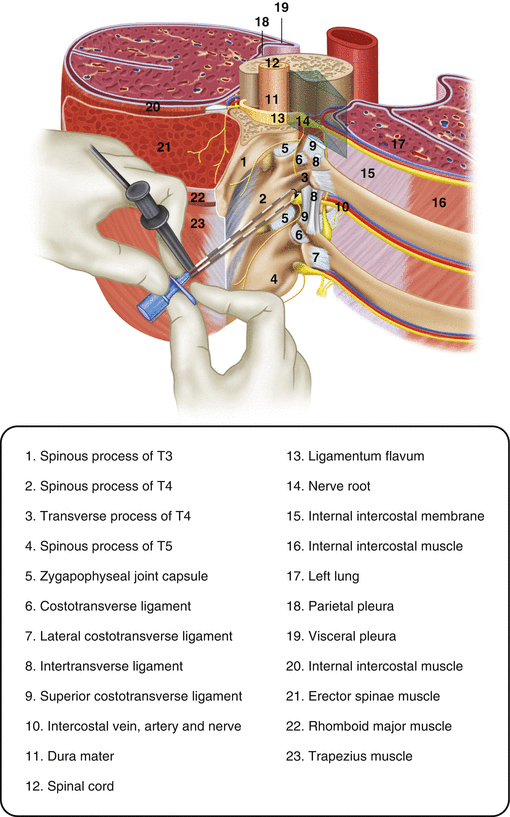
Paravertebral nerve blocks are an alternative modality to epidural catheters. The paravertebral space is a wedge-shaped area on either side of the spine; the paravertebral space is continuous with the intercostal space and the epidural space. Injection of local anaesthetic into the paravertebral space leads to anaesthetic effects not only to the nerve root at the level of the injection but to nerve roots caudally and cranially as well. Paravertebral nerve blocks provide analgesia comparable to epidural analgesia [21] and are considered superior to epidural anaesthesia by some authors [22, 23]. Because paravertebral nerve blocks do not produce hemodynamic instability, they can safely be used in patients with ongoing hemodynamic compromise. Compared to epidural infusions, paravertebral nerve blocks do not produce sensory or motor blocks of the limbs, are not associated with urinary retention, produce less nausea and vomiting and are not associated with the risk of epidural abscess or hematoma [24]. Paravertebral nerve blocks are also safer than epidural catheters in patients receiving anti-platelet therapy and do not require coordination of DVT prophylaxis with epidural insertion or removal. Risks of this technique include pneumothorax and inadvertent epidural injection of analgesic. As with epidural catheters, paravertebral nerve blocks require some degree of patient cooperation. In addition, paravertebral nerve blocks produce analgesia in a smaller distribution than an epidural catheter (Fig. 13.2).