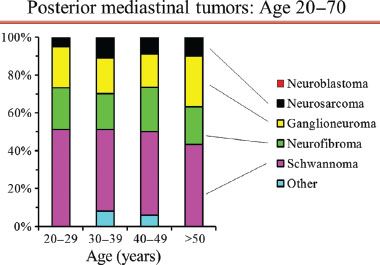
Figure 29.1 Proportion of posterior mediastinal tumor types by decades of age in adults. Legend: N/GN’blastoma, neuroblastoma/ganglioneuroblastoma; PNET, primitive neuroectodermal tumor. Reproduced with permission from Detterbeck: Mediastinal Tumors.32
Benign Lesions
Limited data corroborates the conventional wisdom that benign neurogenic posterior mediastinal lesions are very indolent. Benign tumors have a very low recurrence rate—even if incompletely resected; for example, one recurrence occurring 4 years after an incomplete resection was reported out of 87 resected benign posterior neurogenic tumors.2 Four other incompletely resected benign lesions in this series did not recur during 10 years of follow-up.2 Experts in these tumors have remarked that resection of benign lesions has no influence on patient survival although they recommend resection of benign lesions.2,7
There is limited data on observation of posterior neurogenic tumors in patients deemed to be too high risk for resection. No progression was noted over 5 years of observation in a patient with chronic rejection of a transplanted kidney with foraminal extension of a posterior neurogenic tumor.8 In another series of neurogenic tumors (in various locations, not only thoracic), six tumors that were considered benign based on positron emission tomography (PET) activity were observed and were all found to be unchanged at a median follow-up of 17 months.3
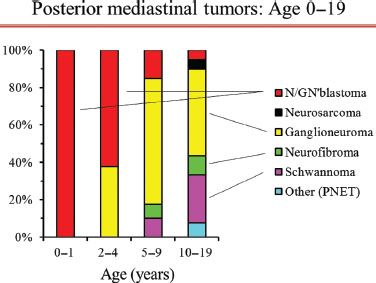
Figure 29.2 Proportion of posterior mediastinal tumor types by age in children and adolescents. Legend: N/GN’blastoma, neuroblastoma/ganglioneuroblastoma; PNET, primitive neuroectodermal tumor. Reproduced with permission from Detterbeck: Mediastinal Tumors.32
In conclusion, anecdotal data suggests that growth occurs in only a minority of benign neurogenic tumors during several years of observation.
Specific Clinical Questions
The traditional recommendation has been to resect all posterior mediastinal neurogenic tumors—even if benign—because of concern about malignant degeneration or complications due to growth. However, to appropriately apply these arguments it is necessary to define how high the risk of these events actually is.
What Is the Risk of Malignant Degeneration?
Papers justifying resection of benign neurogenic tumors because of a risk of malignant degeneration invariably provide no data or references. An extensive literature search revealed no well-documented benign neurogenic tumor that became malignant in adults without neurofibromatosis. Several case reports of malignant degeneration of neurogenic tumors involve either children, tumors of other areas of the body, or tumors not arising spontaneously—for example, after prior irradiation of a neuroblastoma or in an immunocompromised patient.9–11 One case report12 of a thoracic neurogenic tumor in an adult speculated about “malignant degeneration” because it involved a composite tumor with features of ganglioneuroma as well as neuroblastoma; however, this could also be a manifestation of the heterogeneity of tumors or spontaneous maturation from neuroblastoma to ganglioneuroma, as has been described.13
Another case14 involved a malignant neuroblastoma that developed at the site of a previously resected benign retroperitoneal ganglioneuroma 11 years earlier. Local control was achieved with partial reresection and radiation, but eight years later a metastasis at a separate site was found. It was argued that this might represent malignant transformation because it would be unlikely to have had two distinct tumors, and the typically rapid growth of neuroblastoma makes it unlikely, this was a present but missed component in the original tumor. However, this case is very atypical because the indolent behavior (metastasis after 8 years) and the age at diagnosis (age 32) are highly unusual for a neuroblastoma.
Therefore, malignant degeneration of a benign neurogenic tumor is not clearly documented in the literature in patients without neurofibromatosis, and such speculation rests on anecdotal cases of composite tumors and one potential case report in which the malignant tumor exhibited unusual (indolent) behavior. The argument for resection of benign neurogenic tumors because of potential malignant degeneration must be viewed as not being clearly substantiated.
What Is the Risk of Development of Symptoms/Clinical Problems?
An extensive literature search revealed no series of prolonged observation of benign neurogenic tumors that allow estimation of a rate of growth and subsequent development of complications. The lack of case reports of patients eventually resected after initial observation suggests that the rate of growth and development of symptoms is rare. Thus, the potential for clinically significant growth is not well defined but appears likely to be rather low.
A rational argument can be made to resect asymptomatic lesions that involve the spinal canal, given the potentially major consequences if one waits for symptoms to develop. Whether careful surveillance with regular imaging is a safe alternative is not documented in the literature.
What Is the Risk of Malignant Neurogenic Tumor in Patients With Neurofibromatosis?
Patients with familial neurofibromatosis who have intrathoracic tumors present a unique clinical challenge. The lifetime incidence of a malignant peripheral nerve sheath tumor is 2% to 5% in this population, as compared to 0.001% in the general population.15 Nordback et al. suggest a 10% risk of malignancy in intrathoracic neurogenic tumors in patients with neurofibromatosis16; they additionally surmise that surgical trauma may induce malignant transformation based on cases of early appearance of malignant tumors at the site of previously completely resected benign tumors.16 Finally, malignant neurogenic tumors in these patients are said to have higher rates of local recurrence, distant metastases and worse overall survival.17
Making a Clinical Diagnosis
The first step in approaching patients is to establish a presumptive clinical diagnosis and an assessment of how confident one can be of this. The primary clinical issue is knowing when to suspect malignancy. Specific characterization of benign lesions has no clinical relevance. Potential factors to help identify malignancy are reviewed in the next sections.
Demographic Features
Demographic features are of little benefit in defining the nature of a posterior mediastinal mass in adults. Posterior neurogenic tumors occur with similar frequency in all ages from infancy to the eighth decade of life.8 While the probability of malignancy is high in children (∼40% overall, ∼100% if under age 2 years; Fig. 29.2),8 it is 1% to 6% in adults and is relatively constant across all adult ages (Fig. 29.1).7,17
Posterior neurogenic tumors occur equally in men and women; this is true of malignant tumors as well.8 Small differences exist in the incidence of specific benign tumors, but this has no clinical impact (e.g., ganglioneuromas are slightly more common in men and nerve sheath tumors slightly more common in women).2
Medical History
In patients with a posterior mediastinal mass one should specifically ask about a history of neurofibromatosis and look for superficial signs such as café au lait spots or subcutaneous neurofibromas. While neurofibromatosis is associated with 14% of posterior neurogenic tumors overall, it is present in roughly one-half of all cases with malignant tumors.2 In addition, neurofibromas in the setting of neurofibromatosis classically carry a risk of malignant transformation (to be discussed in further detail). However, these rates still imply that in patients with neurofibromatosis the vast majority (∼85%) of posterior mediastinal tumors will be benign.
Symptoms
Posterior mediastinal masses in adults are often detected incidentally on imaging for other reasons. Only 12% to 37% of patients present with symptoms,2,4,8,18 typically related either to neurologic compromise of the spinal cord or local intrathoracic mass effect (chest pain, Horner syndrome, cough, dyspnea, or dysphagia).
Symptoms Suggestive of Malignancy
The presence of symptoms in general is not a significant predictor of malignancy in adults, nor does the absence of symptoms guarantee benignity. (In children, absence of symptoms is a statistically significant predictor of benignity). However, pain or dyspnea specifically was found to be highly indicative of a malignant tumor in adults.2
Symptoms Suggestive of Spinal Involvement
The presence of neurologic symptoms correlates with the likelihood of intraspinal tumor involvement (so-called dumbbell tumors connected by a thin isthmus extending through a neural foramen). However, about 40% of patients with intraspinal tumors have no neurologic symptoms.19,20
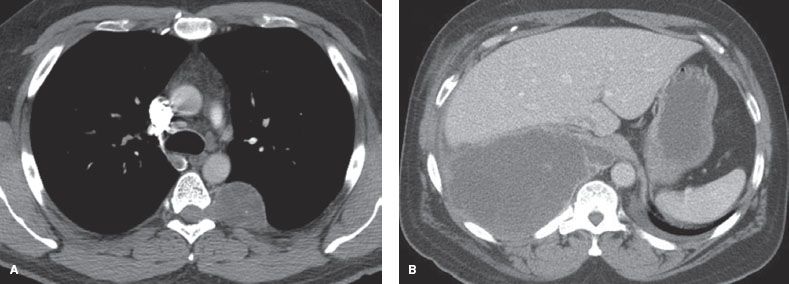
Figure 29.3 Benign and malignant neurogenic tumors. A: Benign neurogenic tumor with bony erosion, widening of the foramen, and extension into the spinal canal. None of these signs are associated with malignancy. B: Ganglioneuroblastoma in an adult. The large size is associated with a likelihood of malignancy.
Imaging
CT
A study of CT imaging of posterior neurogenic tumors in 68 adults identified four imaging features that were associated with malignancy: (a) Size >10 cm (malignancy in almost all patients), (b) bone destruction (four out of four patients), (c) pleural effusion (two out of two patients), and (d) mediastinal displacement (one out of two patients).2 However, while bone destruction was strongly predictive of malignancy, some degree of bony abnormality was noted in 32% (22/68); these findings include erosions, splaying of the ribs, foraminal enlargement, or localized scoliosis and were not associated with malignancy (Fig. 29.3A,B).2
MRI
MRI is an accurate test to assess for a dumbbell tumor.21 Because CT may miss an intraspinal component,20 MRI is indicated whenever the tumor is contiguous with a neural foramen, there is a widened intervertebral foramen or erosion of a vertebral body or pedicle (Fig. 29.4A,B).21 MRI is more sensitive than traditional myelography.20 In addition, magnetic resonance angiography (MRA) can be useful in lower posterior mediastinal tumors to locate the artery of Adamkiewicz instead of arteriography, which has a 2% complication rate.20
MRI features suggestive of malignancy include size >5 cm, prominent vascularity or enhancement, infiltrative margins, marked heterogeneity with central necrosis, rapid growth from a recent imaging study, and increased uptake of Ga-67 citrate.22 However, how well these features predict malignancy is not defined, and MRI is not recommended to categorize posterior mediastinal tumors as benign or malignant.
PET
The role of PET and how to interpret results in posterior neurogenic tumors has not been defined. A retrospective analysis of PET in adult patients found a statistically significantly higher standard uptake value (SUV) in malignant versus benign tumors (median SUV of 2.9 [range 1.8 to 12.3] vs. 1.1 [range 0.5 to 1.8]). A retrospectively applied SUV cutoff of 1.8 yielded a sensitivity of 100% and a specificity of 83%.3 However, in another study of benign tumors in a woman with neurofibromatosis type 1 the SUV ranged from 1.8 to 5.3.23 Further validation is needed to define a specific cutpoint and the false-positive and false-negative rates associated with it. Nevertheless, a strongly positive or negative PET may help confirm a clinical diagnosis and define the extent of disease in some patients.
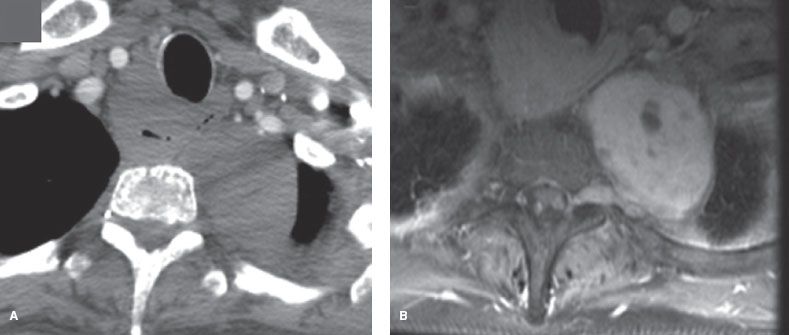
Figure 29.4 Schwannoma in a woman with neurofibromatosis. A: CT with IV contrast does not clearly show invasion through the foramen. B: MRI (T1 postcontrast) image shows invasion through the foramen into the spinal canal.
Laboratory Studies
In children (especially under 5 years) the incidence of neuroblastoma or ganglioneuroblastoma is quite high. Serum vanillylmandelic acid (VMA) or hydroxyl-vanillylmandelic acid (HVMA) was elevated in approximately 30% of malignant neuroblastomas or ganglioneuroblastomas in children.2 The combination of a typical presentation in young children with an elevated serum VMA or HVMA is essentially diagnostic without a biopsy,2 and measurement of these markers in children suspected of having such tumors is clearly justified.
Posterior mediastinal neurogenic tumors in adults are typically nonfunctional.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


