INDICATIONS
Nearly 70% of transplant recipients are between 18 and 49 years old (median age 42 years), with the major indication being congenital heart disease or pulmonary arterial hypertension in adults (Table 4.1). Current cumulative survival data between 1982 and 2011 indicate a 3-month survival of 71%, 1-year survival of 63%, and a 10-year survival of 31% with a 10-year median survival for those surviving the first year after transplant (Fig. 4.3). In contrast, the primary indication for pediatric heart–lung transplantation is cystic fibrosis. Of note, data regarding the survival benefit of heart–lung transplantation for Eisenmenger syndrome remains mixed. Although the long-term survival benefit may be debatable, there is a clear improvement in the quality of life in this group of patients that undergo transplant. Over the last 10 years, there has been a decline in HLTs and an increase in double-lung transplants for cystic fibrosis. Experience shows that normalization of pulmonary pressures following lung transplantation allows for significant recovery of the right ventricle. In patients with noncomplex cardiac defects, repair of the defect combined with single- or double-lung transplantation may be a viable alternative. Also, fewer adults in general are requiring HLTs because of new and improved medical therapies. In younger patients with severe right heart failure and severe pulmonary hypertension, combined HLT is still the best option. Combined HLT may yet see more use as more congenital patients are living longer and reaching adulthood.
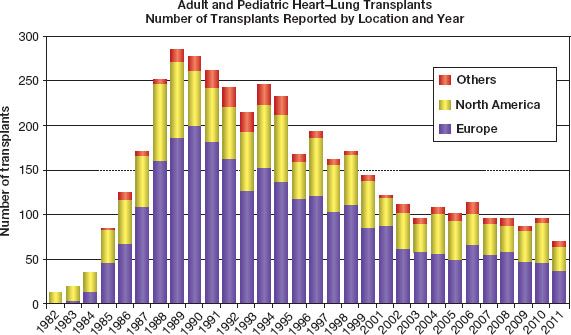
Figure 4.1 Number of transplants reported by location and year.
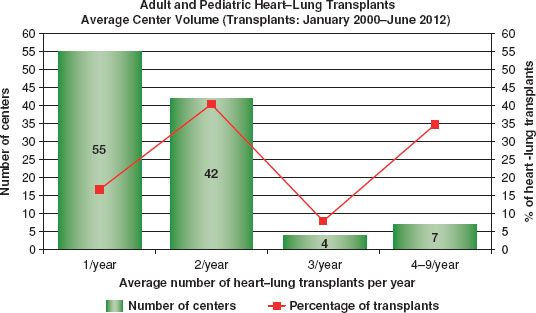
Figure 4.2 Average center volume (transplants: January 2000 to June 2012).
TABLE 4.1 Adult Heart–Lung Transplant Diagnosis (Transplants: January 1982–June 2012)
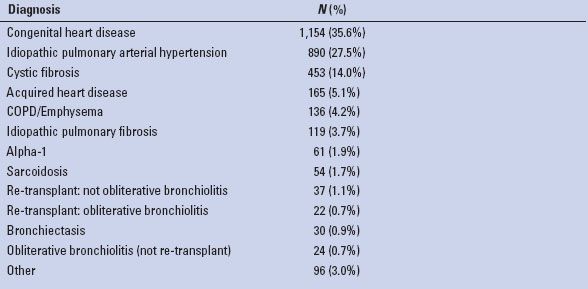
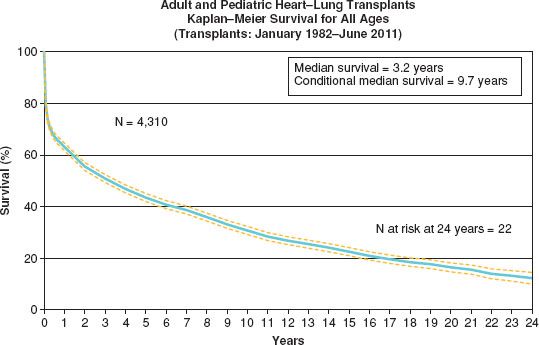
Figure 4.3 Kaplan–Meier survival for all ages (transplants: January 1982 to June 2011).
Patient Selection for Heart–Lung Transplant
As with any surgical procedure or intervention, patient selection is closely linked to outcomes. HLT candidates should meet the established criteria for isolated heart or lung transplantation. The HLT recipient is listed under both heart and lung allocation systems through the United Network for Organ Sharing (UNOS). As in lung transplant, heart–lung candidates are stratified according to their lung allocation score (LAS) and the listing criteria are very similar. In addition, most candidates are New York Heart Association functional class III or IV. The UNOS LAS is meant to allow the sickest patients and the ones with the best chance of recovery post transplant to have priority in receiving organs. The LAS is not comparable to the stratification system used for heart transplant. The LAS also does not clearly reflect the extent of severity of the disease in patients with pulmonary hypertension. These patients may be best served by using the cardiac listing with a special exemption. One criterion that is more stringent in heart–lung than in isolated heart or lung is age. Most centers have an age cut off of 50 years and only a few centers, such as Stanford University, have transplant heart–lung blocs in patients over 60 years of age. With the existing organ shortage, we must do everything possible to ensure a good outcome and prolonged use of the donor organs, especially since each bloc could be used to transplant three different recipients. Another important factor that needs to be considered is recipient–donor size matching. It is critically important not to oversize the heart–lung bloc. As reported by the Papworth Hospital transplant team, attaining predicted total lung capacity at 1 year is to be expected and if not attained then that is suggestive of complications with the transplanted lungs. In the perioperative period, oversizing can lead to tamponade and low cardiac output.
 SURGERY
SURGERY
En Bloc Heart–Lung Recovery
All heart/lung recoveries begin with review of all donor data and pertinent tests, which include serologies, blood gases, CXR, bronchoscopy, chest CT and cardiac catheterization if available. ABO blood type and recipient compatibility should be clearly documented. The donor is placed on the OR table in the supine position with the arms tucked at the sides. The donor height is also rechecked for accuracy especially in an en bloc heart–lung recovery, as size matching is critical. Prior to prepping, a bronchoscopy is performed as days may have passed since the initial examination was performed. Throughout the recovery process, it is best to use low-dose vasoconstrictors, such as Neo-Synephrine or vasopressin for hypotension to minimize the amount of crystalloid that is given. The FiO2 is maintained at 40%. The donor is then prepped and draped up to the sternal notch. A midline sternotomy incision is then performed and continued further cephalad than the usual incision for a heart operation to allow for easier dissection and isolation of the suprainnominate proximal trachea, which is an easier approach than between the aorta and the superior vena cava (SVC). The pericardium is incised down the midline and then laterally down both sides at the level of the diaphragm. Sutures are placed along the pericardial edges and a pericardial well is created prior to entering both pleural spaces. The heart and lungs are visually inspected and palpated. If no abnormalities are appreciated, the recipient team is notified and instructed to proceed. The space between the aorta and pulmonary artery is then dissected with the cautery to allow for x-clamping of the aorta. The SVC is dissected free as cephalad as possible and encircled with a suture, which is then tied prior to x-clamping. The azygos vein is divided at this time. The SVC is also dissected free from the underlying right pulmonary artery. The pericardial reflection around the IVC is released to allow for mobilization. The trachea is then identified and dissected above the innominate vein and between the innominate artery and the left common carotid artery. The trachea is encircled with an umbilical tape. When the abdominal team is ready for x-clamping, 300 International Unit /kg of intravenous heparin is administered. A cardioplegia cannula is placed in the ascending aorta approximately 2.5 cm above the aortic valve. This cannula can be left in place and used for further cardioplegia and de-airing at the time of implantation. A pneumoplegia cannula is then placed in the pulmonary artery at the level of the bifurcation, which is then removed at the time of PA transection. A Satinsky clamp is then placed at the base of the left atrial appendage and the appendage is resected. This site will serve as the venting site for the plegia. The SVC is then ligated, making sure there are no central catheters in place. At this time, 500 mcg of PGE-1 is directly injected into the pulmonary artery next to the cannula. The systolic blood pressure will drop precipitously and at least 1 minute is allowed for the PGE-1 to circulate and then the IVC can be transected. The aortic clamp is placed, the Satinsky is removed from the left atrium, cardioplegia and pneumoplegia are initiated and ice is placed on the heart and both pleural spaces. We prefer to administer 2 L of 4°C Plegisol (Hospira, Inc., Lake Forest, IL) for the heart. For the lungs, we administer 60 mL/kg of 4°C Perfadex (Xvivo Perfusion, Inc., Englewood, CO) at a pressure of 10 to 15 mm Hg. The average-sized donor should receive between 4 and 5 L of pneumoplegia. Since the left atrium is not entered, we do not administer retrograde pneumoplegia, as is customarily done in isolated lung transplant. To avoid over distention, lung inflation is maintained at 50% of total lung capacity, airway pressures at less than 20 cm of H2O and FiO2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


