Category
Disease
Key diagnostic test
Prehepatic
Portal vein thrombosis
CT
Congenital extrahepatic portal vein obstruction
CT
Extrinsic compression of portal vein
CT
Arteriovenous fistula in portal vein system
CT, US
Intrahepatic
Cirrhosis
Clinical Hx, Lab, CT
Congenital liver fibrosis
Liver biopsy
Hepatic sarcoidosis
Clinical Hx, CT, Liver biopsy
Idiopathic portal hypertension
Liver biopsy
Posthepatic
Budd-Chiari syndrome
US, Hepatic venogram, Hypercoagulable work-up.
Venoocclusive disease
US, Hepatic venogram, Liver biopsy
Prehapatic portal hypertension indicates blockage of the portal or mesenteric vein, mainly due to portal vein thrombosis (PVT). Underlying causes of PVT include liver cirrhosis, tumor thrombus, and, rarely, chronic pancreatitis.
Intrahepatic portal hypertension includes liver cirrhosis and hepatic fibrosis. This can result from a wide variety of etiologies including viral and autoimmune hepatitis, alcohol abuse and toxicity, nonalcoholic steato-hepatitis (NASH), biliary tract disease such as primary biliary cirrhosis and sclerosing cholangitis, as well as a score of less common diseases.
Posthepatic portal hypertension indicates outflow blockage from the liver. Budd-Chiari syndrome implies thrombosis or sclerosis of the hepatic veins and/or the inferior vena cava (IVC). Fifty percent of Budd-Chiari syndrome is associated with an underlying chronic myeloproliferative disorder (e.g., polycythemia vera, essential thrombocythemia) and an accompanying hypercoagulable state. Work-up for the underlying hematologic disease is necessary. Veno-occlusive disease (VOD) is the occlusion of the terminal hepatic venules and hepatic sinusoids associated with hematopoietic cell transplantation and less commonly with liver transplantation, chemotherapeutic agents, and alkaloid toxins.
Diagnosis
Physical Exam
Ascites is the most common physical finding of patients with portal hypertension. Massive ascites occurs most often in uncompensated portal hypertension. As the body attempts to decompress the elevated pressure, there is a natural response of collateral formation. One type of superficial portosystemic shunt, the caput medusa, can sometimes be seen radiating from the umbilicus as a collateral form from the umbilical vein. A physical examination should include a rectal exam to check for hemorrhoids and the presence of frank or occult blood that can be caused from bleeding from varices in the esophagus, stomach, rectum, and less commonly, other areas of the GI tract. Altered mental status, hepatic encephalopathy, can be seen due to the existence of well-developed, large portosystemic shunts. Current status and history of encephalopathy and the need for lactulose or rifaximin must be assessed carefully since encephalopathy can be exacerbated after the surgical or radiological creation of a new shunt. Uncontrolled encephalopathy is a contraindication for these procedures.
Laboratory
Degree of Portal Hypertension
Thrombocytopenia, along with splenomegaly, is correlated with the degree of portal hypertension. This is known as hypersplenism. Anemia is a common finding in these patients due to chronic liver disease or gastrointestinal bleeding.
Scores to Determine Treatment Options
The Child-Pugh score is widely used to quantify hepatic function reserve, predict prognosis, and select the appropriate surgical intervention. Mortality rates following open abdominal surgery have been reported as 10 %, 17–31 %, and 63–82 % respectively for the patients with Child A, B, and C [1–3]. The Model for End-stage Liver Disease (MELD) score, calculated with total bilirubin, PT-INR, and creatinine, was originally developed to predict the prognoses of patients who undergo Transjugular Intrahepatic Portosystemic Shunt (TIPS) surgery. The MELD score is predictive of 3-month mortality of cirrhotic patients and has been used in the liver allocation system for liver transplant candidates in many countries. It has been suggested that patients with a MELD score below 10 may undergo elective surgery and those with a MELD score greater than 15 should not undergo elective surgery [4].
Hypercoagulable Status
Patients with portal vein thrombosis should be evaluated for hypercoagulable status, including, but not limited to, tests for serum level of protein C, protein S, and antithrombin III, as well as genetic tests for factor V Leiden and JAK-2 gene mutations.
Imaging
Endoscopy
The endoscope is a valuable tool for the screening and treatment of bleeding or nonbleeding varices.
Bleeding varices can be treated with injection sclerotherapy or more commonly variceal band ligation. Band ligation is somewhat more difficult to perform, but has lower morbidity than sclerotherapy and has now become the first-line treatment of choice.
Hepatic Doppler Ultrasonography (US)
Hepatic Doppler US can be used for the screening of thrombosis in the portal and hepatic veins. For the diagnosis of Budd-Chiari syndrome, US has a sensitivity and specificity of 85–90 %. Findings on US consistent with Budd-Chiari syndrome are the absence of flow or thrombosis in the hepatic veins, obstruction of the retrohepatic IVC, and a hypertrophic caudate lobe.
Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI) with Contrast
Triphasic CT with iodine contrast is the best modality to visualize the global picture of the portal venous system and to evaluate the extension of portal vein thrombus. MRI with gadolinium contrast is an alternative for patients who have a history of an allergic reaction to iodine. Compared to an angiogram, CT and MRI provide 3-dimensional information of the anatomy of collateral veins related to adjacent organs and are useful for determining the surgical plan. While contrast CT or MRI can visualize detailed anatomy of collateral vessels, they do not provide information regarding flow volume or direction. In order to evaluate actual flow in collateral vessels, a dynamic angiogram is necessary.
Noncontrast Magnetic Resonance Angiogram (MRA) Without Gadolinium
For patients with kidney dysfunction, image modality options are limited. Triphasic CT with iodine contrast can be performed only when kidney dysfunction is mild and after the patient has had appropriate hydration. However, the administration of iodinated contrast risks further deterioration of renal function due to contrast-induced nephropathy. Additionally, gadolinium contrast for MRI is known to cause irreversible nephrogenic systemic fibrosis, the incidence of which has been reported as up to 7 % for the patient with GFR <30 %.
In these circumstances, noncontrast MRA can help assess the extension of thrombus and the anatomy of collaterals without intravenous administration of gadolinium [5]. Non-contrast MRA provides decent quality, although ascites causes some artifact (Fig. 8.1).
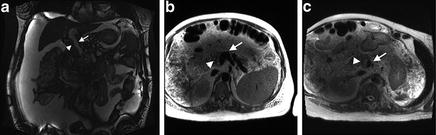

Fig. 8.1
Noncontrast MRA was performed in a patient with kidney dysfunction to assess the extent of portal vein thrombus and the patency of the confluence of splenomesenteric confluence. (a) True fast imaging with steady state precession (TrueFISP): Fast flowing blood is bright and stationary thrombus is dark. Arrowhead indicates nonocclusive adherent thrombus. Arrow indicates patent portion of main portal and left portal veins. (b, c) Single-Shot Fast Spin Echo sequence (HASTE): Fast flowing blood is dark and stationary thrombus is bright. Arrowheads indicate nonocclusive adherent thrombus. Arrows show patent splenic and superior mesenteric venous confluence.
Visceral Angiogram with Portography
A dynamic visceral angiogram through the celiac, superior mesenteric, and inferior mesenteric arteries with a late phase portography is performed to assess the flow pattern of the portal vein system. With portal hypertension, main branches of the portal vein system (portal, splenic, gastric, and mesenteric vein) may be separated from each other or connected with little communication due to thrombus or abnormal anatomy. When there are several collateral veins and portosystemic shunts (e.g., spleno-renal shunt, spleno-retroperitoneal shunt, and gastroesophageal varices), an angiogram with a portogram can show which shunt is the dominant route of collateral flow.
Hepatic Venogram with Pressure Measurement/Transjugular Liver Biopsy
Wedged hepatic venous pressure (WHVP) reflects the hepatic sinusoidal pressure and therefore the portal pressure. The hepatic venous pressure gradient (HVPG), as measured by hepatic venography, is the pressure gradient between the WHVP and the free hepatic venous pressure, and thus is an estimate of the pressure gradient across the liver.
HVPG measurement is useful to differentiate between prehepatic (normal HVPG), hepatic, and posthepatic causes of portal hypertension (HVPG > 5 mmHg). Additionally, the cavogram with the hepatic venogram is a gold-standard modality to diagnose the Budd-Chiari syndrome and VOD. A hepatic venogram may demonstrate a “spiderweb” pattern diagnostic of Budd-Chiari syndrome. Liver biopsy is nonspecific for Budd-Chiari syndrome, but required for a definite diagnosis of VOD.
Management
Recent advances in the medical and endoscopical management of variceal bleeding have shifted from surgical to nonsurgical treatments. However, failure of medical treatment requires interventions such as radiological or surgical shunt creation. These interventions are both palliative and life-saving. Liver or multivisceral transplantation is the ultimate treatment to follow other treatment failures and for patients with Child C cirrhosis [6]. The suggested management algorithm for variceal bleeding is summarized in Fig. 8.2.