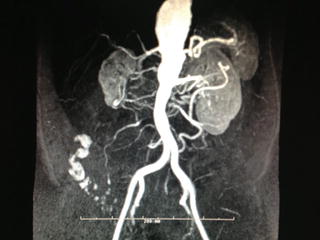
Fig. 10.1
Magnetic resonance imaging demonstrating a 5.0 cm thoracic aortic aneurysm in a patient with long-standing giant cell arteritis.
Pathophysiology
The cause of GCA remains unknown. Evidence from the laboratory supports that GCA is an antigen-driven disease in which macrophages, dendritic cells, and T lymphocytes play a critical role.
Diagnosis
The diagnosis of GCA is made based on compatible clinical features together with evidence supporting vasculitis from either by temporal artery biopsy or large vessel imaging.
Physical Exam is very important in GCA. Head and neck examination should include examination of the scalp and tongue for areas of ischemia and palpation of the temporal arteries for tenderness, nodularity, and pulse. Ocular examination should include assessment of pupillary reactivity and extraocular movements, and any patient with visual symptoms should be referred immediately to ophthalmology for evaluation. A careful vascular examination should be performed to look for signs of large vessel involvement including four extremity blood pressure measurements, examination of peripheral pulses, auscultation for bruits, and abdominal palpation for assessment of the aortic pulse and dimension. Cardiac examination should include auscultation for murmurs. Musculoskeletal examination may reveal tenderness over the deltoid and trochanteric areas and swelling of the peripheral joints can be seen in some patients with PMR.
Laboratory findings are nonspecific and there are no laboratory findings that are diagnostic for GCA. Leukocytosis, anemia, thrombocytosis, and elevation of the ESR and/or CRP are typically seen consistent with any inflammatory process. GCA is not associated with ANCA.
Imaging:
Plain film. Chest radiograph can provide information about the thoracic aorta. It has been used in some instances as a screening tool to monitor for the development of thoracic aortic aneurysms in patients who have other GCA features but may not be sufficiently sensitive to detect small aneurysms.
Duplex ultrasound has been used to examine the temporal arteries with description of the “halo sign” as an indicator of vascular inflammation. The utility of temporal artery ultrasound in GCA has been controversial. Although some studies from centers where ultrasound is commonly performed have found this to be useful in guiding biopsy and supporting the diagnosis, other studies have not found this to be any more effective than a thorough physical examination [15, 16]. As the utility of temporal artery ultrasound is heavily influenced by user technique and experience, it is unlikely to have a role in most clinical practices and should not be used as a means of confirming a diagnosis of GCA at this time.
Computerized CT angiography (CTA). CTA can be used to noninvasively visualize the aorta and branch vessels and can provide supportive evidence of large vessel vasculitis. CTA requires contrast and X-ray exposure but remains the preferred technique by some vascular surgeons.
Magnetic resonance angiography (MRA). MRA can also be used to noninvasively visualize the aorta and branch vessels and can provide supportive evidence of large vessel vasculitis. MRA has also recently been used to visualize the temporal arteries. Fat saturated spin echo or gradient echo sequences have been used to examine the vessel wall. While vessel enhancement has been observed in conjunction with edema and inflammation, in Takayasu arteritis this was not uniformly reliable, particularly over serial imaging. At this time, the degree or change in vessel enhancement by MRI should not be used as a measure of disease activity.
Angiography is uncommonly used in GCA due to the availability of noninvasive large vessel imaging techniques via CTA or MRA. Angiography may still have a role, however, when central blood pressure measurements are needed in patients who have four-extremity stenotic disease making peripheral measurements unreliable or for endovascular intervention.
Fluorodeoxyglucose positron emission tomography (FDG–PET). FDG-PET is a noninvasive imaging technique that visualizes metabolically active tissues. Although primarily used in neoplastic and infection disease settings, large vessel uptake has also been found in GCA. The utility of FDG-PET in GCA remains unclear at this time and is being further investigated.
Histology
Temporal artery biopsy represents the primary means of diagnosing GCA. Biopsy would typically be performed on the symptomatic side, obtaining 2–3 cm of artery. Unfortunately, the positive diagnostic yield of temporal artery biopsies ranges from 50 to 80 % and is even lower in those patients with large vessel presentations. The diagnostic yield may be slightly increased by performing bilateral temporal artery biopsies, but this remains an individual physician decision as to their preference of performing unilateral or bilateral biopsies. In patients where there is a high degree of suspicion for GCA, treatment with glucocorticoids should not be delayed pending the biopsy as histologic changes have been found to persist for weeks after treatment has been started. The histologic findings in GCA include mononuclear cell infiltration involving the adventitia, media, and intima (Fig. 10.2). Multinucleated giant cells, although part of the disease nomenclature, are seen in only 50 % of cases and tend to group around the disrupted elastic lamina.
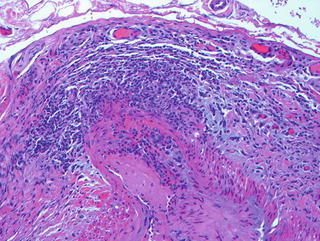

Fig. 10.2
Temporal artery biopsy in a patient with giant cell arteritis demonstrating panmural inflammation.
Differential Diagnosis
The differential diagnosis of GCA is based upon its clinical features. For cranial arteritis this can include thromboembolic disease, other causes of headache, and vision loss. In patients with PMR, the main differential can include rheumatoid arthritis, osteoarthritis, fibromyalgia, and polymyositis. For large vessel disease, this differential would be as described for Takayasu arteritis.
Management
Glucocorticoids represent the foundation of treatment for GCA and are typically combined with low-dose aspirin. Unfortunately, glucocorticoids are associated with a high rate of toxicity and are often required for extended periods of time. To date, no other immunosuppressive agents have been identified as being effective in GCA to treat the disease, reduce glucocorticoid requirements, or prevent relapse. Surgical intervention can play an important role in the setting of large vessel disease.
Immunosuppression
Glucocorticoids. Since the 1950s, glucocorticoids have been recognized as being effective in reducing not only the symptoms of GCA but more importantly the risk of blindness. Shortly after the introduction of glucocorticoids for the treatment of GCA, the risk of bilateral blindness went from 17 to 9 % and current studies suggest the risk of blindness after starting glucocorticoids to be only 1 % [17]. For this reason, any patient where the diagnosis of GCA is suspected should be started on glucocorticoids immediately to protect vision while the diagnostic evaluation is being pursued. The usual starting dose of prednisone is 40–60 mg/day. This dose is continued for 4 weeks then reduced slowly. There is no widely accepted standard means to reduce prednisone, although one schedule includes a reduction by 5 mg every 1–2 weeks until reaching 20 mg/day then by 2.5 mg every 1–2 weeks until 10 mg/day then by 1 mg every 2–4 weeks. For patients who present with a transient or fixed vision loss, methylprednisolone 1,000 mg/day for 3 days is often given as the initial glucocorticoid dose followed by prednisone 40–60 mg/day. Unfortunately, once vision loss has occurred it is very unlikely to return and the goal of using a methylprednisolone pulse is to protect vision in the remaining eye.
Aspirin. In two retrospective studies, the use of aspirin was found to be associated with a lower rate of cranial ischemic complications (blindness, stroke) [18]. Although this has not been proven in a prospective trial, the current data supports the use of aspirin 81 mg/day as an adjunctive therapy to prednisone in all patients with GCA who do not have a contraindication.
Other immunosuppressive agents. Because of the degree of toxicity associated with long-term glucocorticoids, alternative agents have been pursued that reduce the need for glucocorticoids and that reduce relapse. Randomized trials examining methotrexate did not reduce the rate of glucocorticoid toxicity [19]. Infliximab, a monoclonal antibody directed against tumor necrosis factor (TNF), was found to be no more effective than placebo in reducing relapses and was associated with an increased risk of infection [20]. To date, there remains no therapy beyond glucocorticoids that has been found to be effective in GCA.
Isolated aortitis. There are instances where a patient with a thoracic aortic aneurysm of sufficient size is taken to surgery and unexpectedly found on tissue histology to have evidence of aortitis [21, 22]. In such instances, a careful history should be taken for past or current features of GCA or PMR, acute phase reactants should be measured away from the time of surgery, and vascular imaging by CTA or MRA performed to look for disease in other locations. If there is no evidence by this evaluation to suggest a past or current underlying systemic vasculitis, this would be consistent with a form of single organ vasculitis isolated to the aorta. Such patients with focal idiopathic aortitis may not require glucocorticoids, with the surgical intervention that they have already had being the only required treatment. Such patients should be referred to rheumatology for a thorough evaluation to assure the absence of systemic disease that would require glucocorticoids and for ongoing monitoring.
Nonmedical intervention. Nonmedical interventions in GCA are primarily used to address aortic aneurysms for stenotic lesions causing severe symptoms [23, 24]. Unless intervention is absolutely necessary to protect life or vital organ function, vascular procedures should be avoided when active vasculitis is present and deferred until medical treatment has reduced vessel wall inflammation. The role of perioperative glucocorticoids in clinically quiescent patients remains unclear.
Aortic aneurysm grafting. In GCA, aortic aneurysm rupture or dissection can occur with smaller size aneurysms if disease activity persists and aneurysms can progress rapidly [23]. The choice of surgical techniques to address aortic aneurysms in GCA does not differ from patients without GCA and depends on the extent of disease. However, involvement of aortic branches in GCA may be more distal, which may increase postoperative complications.
Peripheral vascular surgery. Intervention for upper extremity stenotic lesions should be performed only for extreme claudication affecting quality of life, given the potential for collateral circulation to develop in this location.
Endovascular. Endovascular aortic aneurysm repair (EVAR) can be safely performed in selected patients with GCA [23]. There is less experience with the use of endovascular techniques such as stenting or angioplasty in GCA, but is likely to have low long-term patency rate based on the experience in Takayasu arteritis.
Complications
Mortality. Life expectancy in patients with GCA has been found to remain the same as the general population. Thoracic aortic aneurysms, however, are associated with an increased risk of mortality.
Organ damage. Despite effective therapeutic intervention, permanent organ damage can result from irreversible injury that occurred during the initial period of active vasculitis. Once vision loss has occurred, it is very unlikely to return.
Treatment–related complications. Treatment for GCA requires the use of glucocorticoids, often for extended periods of time. Up to 86 % of patients with GCA have been found to experience one or more glucocorticoid-related adverse events. The older patient population who develop GCA are particularly susceptible to glucocorticoid toxicities that include infections, bone loss, cataract formation, myopathy with increased risk of falls, diabetes, and hypertension.
Follow-Up
Patients with GCA require frequent laboratory and physician monitoring to follow the response to treatment, to minimize treatment-related complications, and to watch for evidence of relapse. Relapse requiring an increase or reinstitution of glucocorticoids occurs in 70–90 % of patients with GCA. While this often presents with PMR, cranial, or inflammatory symptoms, late onset large vessel disease represents a potential relapse manifestation of GCA. In patients with known large vessel disease, vascular imaging by CTA or MRA should be performed every 6–12 months and for the development of new clinical features. For patients who do not have large vessel involvement, the optimal monitoring beyond physical examination remains unclear, although annual chest radiography to monitor the thoracic aortic shadow has been advocated by some investigators.
Takayasu Arteritis (TAK)
Takayasu arteritis (TAK) is defined as an arteritis, often granulomatous, predominantly affecting the aorta and/or its major branches, with a predilection for the branches of the carotid and vertebral arteritis with an onset in patients younger than 50 [1]. TAK is an uncommon disease occurring in three per one million people. As defined, TAK occurs in people younger than the age of 50 years with an average age in the 20s. There is a 9:1 female predominance in patients from Japan and the United States, although nearly equal representation in men and women has been seen in other countries. TAK may have a varying spectrum in different populations but is seen throughout the world.
Clinical Presentation
In 10–20 % of patients with TAK, there are no presenting features and evidence of disease is found incidentally. The remaining 80–90 % of patients present with features of systemic inflammation (fever, weight loss, malaise, night sweats) and/or vascular symptoms. Vascular symptoms occur as a direct reflection of the location of the stenotic or aneurysmal disease and the tissues being provided by this blood flow [25, 26]. The subclavian artery is usually the most commonly affected vessel during the patient’s course (20–78 %) with stenosis resulting in upper extremity claudication. Renal artery involvement (15–50 %) can result in hypertension and renal insufficiency (Fig. 10.3). Involvement of the carotid and vertebral arteries can manifest with central nervous system hypoperfusion symptoms including transient ischemic attack (TIA), stroke, syncope, visual changes, dizziness, and hearing loss. Involvement of mesenteric vessels is common but frequently asymptomatic due to collateral flow. Thoracic aortic aneurysms can result of aortic root dilation and aortic valvular insufficiency.