Pneumothorax
DEFINITION
The abnormal presence of air in the pleural cavity, separating the visceral from the parietal pleura, with subsequent collapse of the adjacent lung, is known as pneumothorax. Traditionally, pneumothorax has been classified as having occurred spontaneously or as a result of trauma. Spontaneous pneumothorax is then further subclassified as having occurred without an apparent cause, in a background of clinically normal lungs (primary spontaneous pneumothorax [PSP]) or having occurred in the setting, and as a consequence of, underlying lung disease (secondary spontaneous pneumothorax [SSP]). Although it is now believed that the majority of PSPs occur in the setting of occult lung disease with underlying anatomical abnormalities such as subpleural blebs, this method of classification is still in common practice.
Traumatic pneumothorax occurs as a result of either blunt or penetrating trauma to the chest with a subsequent disruption of the structural integrity of the lung, chest wall, esophagus, trachea, or bronchi. Iatrogenic pneumothorax is a subtype of traumatic pneumothorax resulting as a consequence of diagnostic or therapeutic procedures such as central-line insertions, thoracentesis, or mechanical ventilation.
PATHOPHYSIOLOGY
As alluded to above, air does not normally exist within the pleural space. The pressure within the pleural space is negative with respect to the alveolar pressure during the entire respiratory cycle. Despite this, air does not enter the pleural space from the alveoli. The reason for this lies in the fact that the partial pressure of all gases in the venous blood averages only 706 mm Hg, thereby driving the net movement of alveolar air (760 mm Hg at sea level during end-inspiration) into the capillaries and not into the pleural space, which would require a pleural pressure lower than 706 mm Hg to pull the air in. Under normal circumstances, most individuals do not generate a net negative inspiratory force of –54 mm Hg.1
The negative pressure in the pleural space results from the inherent tendency for the lung to collapse (elastic recoil) and of the chest wall to expand. The negative intrapleural pressure is not uniform throughout the pleural space; a gradient of 0.25 cm of water per centimeter of vertical distance can be measured between the apex and the base of the lung. At the apex, the pressure is more negative than at the base, and this pressure difference tends to favor a greater distention of the alveoli located in this region.
The presence of air in the pleural cavity is thought to occur by one of three events: a communication between the pleura and the alveolus, a communication between the atmosphere and the pleural space, or the presence of a gas-producing organism within the pleura.1 When a communication develops between an alveolus and the pleural space, air will move from the alveolus into the pleural space until there is equalization of pressure or until the communication is sealed. The same happens with a communication between the chest wall and the pleural cavity. The greater the amount of air that enters the pleural cavity, the more the lung collapses. If unabated, enough air may enter the pleural space to cause a shift of the mediastinum and eventually lead to hemodynamic compromise, a condition referred to as tension pneumothorax.
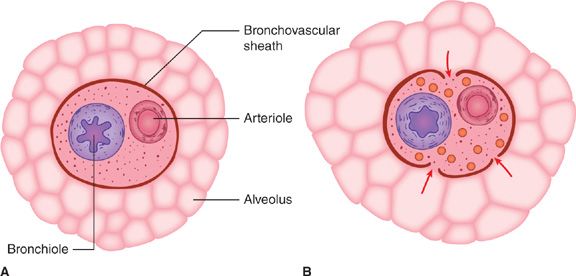
Although the mechanism responsible for PSP is not completely understood, experimental overdistention of normal lungs results in rupture of subpleural alveoli.2 Air can dissect along the bronchovascular sheath medially to produce pneumomediastinum, which may be accompanied by subcutaneous emphysema or pneumothorax (Fig. 78-1), or it can dissect to the peripheral portion of the lung.3 Peripheral dissection of air may result in an air-containing space within or immediately beneath the visceral pleura. Pathologic studies of resected lungs from patients with PSP usually show one or both of these types of airspaces, a bleb or a bulla. A pneumothorax may occur when these peripheral bullae or blebs become distended and rupture into the pleural space.
Figure 78-1 Proposed mechanism of alveolar rupture in spontaneous pneumothorax. A. Normal structures. B. Overdistention of marginal alveoli. Pressure in the adjacent bronchovascular sheath remains lower than in the overdistended alveoli. This pressure gradient may lead to rupture of the alveoli with dissection of air toward the pleura or mediastinum. (Reproduced with permission from Maunder RJ, Pierson DJ, Hudson LD. Subcutaneous and mediastinal emphysema: pathophysiology, diagnosis, and management. Arch Intern Med. 1984;144(7):1447–1453.)
Clinically, the results of a pneumothorax are dependent on the degree of lung collapse but the main physiologic consequences of a pneumothorax are a decrease in the vital capacity of the lung and a decrease in the PaO2. The reduction in arterial PaO2 appears to be caused by low ventilation–perfusion (V/Q) ratios, anatomic shunts, and, occasionally, alveolar hypoventilation. Total lung capacity, functional residual capacity, and diffusing capacity are also reduced, though less than vital capacity. Air in the pleural space eliminates the gravitational gradients of pleural pressure and regional lung volume so that regional ventilation is more uniform and reduced. Anthonisen reported that lungs demonstrate airway closure at low lung volumes and suggested that airway closure is the main cause of V/Q imbalance in patients with pneumothorax.4 If perfusion to the collapsed lung is preserved, there is an increase in pulmonary shunt and substantial hypoxemia. If perfusion to the collapsed lung is reduced by hypoxic vasoconstriction, hypoxemia may be minimal. In general, pneumothoraces occupying less than 25% of the hemithorax are not usually associated with significant shunts.5 Under normal circumstances, despite the degree of pneumothorax; hypoxemia tends to abate within 24 hours, presumably from redistribution of pulmonary blood flow.
In the healthy person, the decrease in vital capacity and PaO2 is typically well tolerated. In patients with compromised pulmonary function prior to pneumothorax, the decrease in vital capacity may result in significant hypoxemia, alveolar hypoventilation, and respiratory acidosis. Upon evacuation of air from the pleural space, the PaO2 usually improves. In animal studies, the PaO2 returns to baseline immediately after reexpansion of the lung.6 In humans, normalization of the PaO2 takes longer and may occur over hours to several days. The delay in improvement may be related to the duration of the pneumothorax.
REABSORPTION OF PLEURAL GASES
The time required to absorb all gases in a pneumothorax is quite variable. It has been estimated that between 1% and 6% of a pneumothorax is absorbed in 24 hours.7 Gas reabsorption from the pleural space is achieved by simple diffusion from the pleural space into the venous blood. The rate of gas reabsorption depends on four variables: (1) the pressure gradient for the gases between the pleural space in relation to the venous blood, (2) the diffusion properties for the gases present in the pleural space, (3) the area of contact between the pleural gas and the pleura, and (4) the permeability of the pleural surface. (i.e., a thickened, fibrotic pleura will absorb less than normal pleura.)
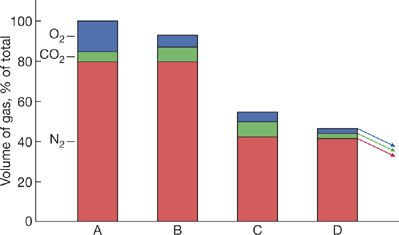
The solubility and diffusion properties of different gases vary considerably, and the speed of reabsorption will depend on the type of gas. Oxygen will be absorbed 62 times faster than nitrogen, the slowest gas to be reabsorbed. Carbon dioxide will be absorbed 23 times faster than oxygen, and carbon dioxide and water vapor will equilibrate almost instantaneously. Under normal circumstances, the total gas pressure in the pneumothorax is within a few millimeters of mercury of that of the atmosphere, or 760 mm Hg. Tissue gas tensions are close to those of systemic venous blood: typically pCO2 = 46 mm Hg, pO2 = 40 mm Hg, pH2O = 47 mm Hg, and pN2 = 569 mm Hg, giving a total pressure of 702 mm Hg as mentioned above. This positive-pressure gradient between the pneumothorax and the venous blood constitutes the driving force responsible for gas reabsorption from a pneumothorax.
When breathing room air, this driving force is only 58 mm Hg (760–702 mm Hg) and this limits the rate of resorption (Fig. 78-2). During inhalation of 100% oxygen, the total gas pressure is about 146 mm Hg. This difference is due to the reduction in partial pressure of nitrogen from 569 to zero mm Hg. Though this is associated with an increase in oxygen in arterial blood from 100 to 640 mm Hg, oxygen consumption in the tissues ensures that the partial pressure in the end-capillary blood rises only slightly (from 40 mm Hg to about 54 mm Hg). The results of one, small prospective human trial and one experimental, animal model trial suggest that administration of high-flow oxygen can expedite resolution of a pneumothorax.8,9
Figure 78-2 Hypothetical representation of the resorption of a spontaneous pneumothorax. A. Closed pleural space after leak has stopped. The alveolar gas in the space contains 15% O2, 5% CO2, and 80% N2. B. CO2 and O2 have quickly equilibrated with the surrounding tissues; the amount of N2 in the pneumothorax is unchanged. The pneumothorax is already decreased by about 10%. C. The number of N2 molecules is unchanged, but the total volume of the pneumothorax has decreased (see B). Therefore, the outward diffusion of N2 increases because pN2 in the pleural space is greater than pN2 in the tissues. As N2 diffuses out, the total volume of gas in the pleural space decreases and concentrations of O2 and CO2 increase. As a result, O2 and CO2 diffuse out of the pleural space. D. The high N2 concentration promotes the exit of N2 from the pleural space and continues the cycle by which the pneumothorax grows smaller. (Reproduced with permission from Farhi LE. Atmospheric nitrogen and it’s role in modern medicine. JAMA. 1964;188:984–993.)
SYNDROMES
 PRIMARY SPONTANEOUS PNEUMOTHORAX
PRIMARY SPONTANEOUS PNEUMOTHORAX
Epidemiology
The age-adjusted annual incidence of PSP ranges from 7.4 to 18 cases per 100,000 population in males and 1.2 to 6 cases per 100,000 in females. The male-to-female ratio ranges from 3:1 to as high as 6:1 and the characteristic patient is a tall, thin male in the third to fifth decade of life.1,10
Patients with PSP tend to be taller and thinner than control populations. A study of military recruits who developed spontaneous pneumothorax found subjects to be, on average, 2 in taller and 25 lb lighter than the typical military recruit.11 In another study, the frequency of PSP rose with increasing stature and reached an incidence of 200 per 100,000 person-years for subjects at least 76 in tall.10
In addition to stature and male sex, tobacco smoking significantly increases the risk of spontaneous pneumothorax and has been associated with a ninefold or greater risk of developing a first PSP.12 The relative risk of PSP has been shown to exhibit a dose–response relationship with the quantity of cigarettes per day and the length of exposure, increasing more than 20 times in men who smoke one-half pack per day and 100 times in men who smoke one pack per day compared to nonsmokers. The lifetime risk in healthy smoking men may be as high as 12%, as opposed to 0.1% in nonsmokers.12 One review of 402 patients with spontaneous primary pneumothorax reported that 92% of the patients were smokers or ex-smokers.5 Another study showed that patients who had stopped smoking more than 1 year before their first spontaneous pneumothorax had no recurrence during a follow-up of 5.2 years.13
Etiology
Although the diagnosis of PSP is restricted to patients without primary lung disease, it is likely that many of these patients do in fact have underlying lung pathology. Thus, a more accurate description of PSP is pneumothorax occurring in patients with no obvious lung disease.14 While the exact mechanism by which a spontaneous communication between the alveolar space and the pleura has not been elucidated, most authors believe that rupture of a previously unidentified bleb or bulla adjacent to the pleura is to blame for the development of PSP.15 These subpleural blebs and bullae are typically referred to as emphysema-like changes (ELCs) when seen on CT scan and, although it has been shown that the majority of patients with PSP have ELC, no causal relationship has clearly been established. These blebs are demonstrated by chest radiograph in only 20% of cases of PSP, but can be seen in approximately 80% of affected patients by both CT imaging and by surgical evaluation.16,17 In two studies, ELCs were found in 89% on the ipsilateral side and up to 80% bilaterally, while only 20% of those without PSP had these changes.18,19 Another study showed that 81% of nonsmokers with healed PSP had ELCs while those nonsmokers without PSP had none.20 Despite this clear association, a causative relationship is still debated. In part, this is due to the observation that only a minority of blebs are observed to be ruptured at the time of surgery, and other lesions, termed pleural porosity, are observed.1 Pleural porosity is believed to predispose the pleura to air leakage by disruption or replacement of the mesothelial cells of the visceral pleura with inflammatory cells that increase pleural permeability.17
The pathogenesis of these bullae, blebs, and pleural porosity is not definitively known but has been associated with a variety of elements, most commonly with airway inflammation.21,22
Airway inflammation secondary to cigarette smoking may be associated with or contribute to the development of these blebs. Respiratory bronchiolitis in smokers may be an important element in the development of PSP.23 Pathologic evidence of respiratory bronchiolitis was found in more than 88% of smokers undergoing surgery for PSP.24
In addition to airway inflammation, other factors have been linked to ELC and pleural porosity, including connective tissue abnormalities, hereditary influences, increased negative pressure at the apices associated with ectomorphic body habitus, anatomical bronchial abnormalities, and overdistention of alveoli with poor collateral ventilation. Pleural pressure is most negative at the apices, and the degree of negativity relates to the height of the lungs. The alveoli of taller persons are subjected to greater mean distending pressures. Over a long period, this phenomenon could lead to the formation of subpleural blebs in a taller population genetically predisposed to bleb formation.
There are multiple reports of genetic associations or patterns of PSP. Some reports suggest that PSP is inherited through an autosomal dominant gene with variable penetrance25 while others report an associated autosomal recessive26 or an X-linked recessive inheritance pattern.25 Genetic risk factors that have been associated to PSP include the HLA haplotype A2B40,27 the α1-antitrypsin phenotypes M1M2, and the FBN1 gene mutations.28
The rate of recurrence after a PSP is approximately 25% (range: 23%–52%) and recurrence usually occurs within 1 to 2 years after the first episode.29 The rate of recurrence may increase with each successive pneumothorax. In one study, the risk of recurrence increased to more than 60% after the second pneumothorax and to 83% after the third.30 Although there is no predilection for the right or left hemithorax with the initial episode, more than 75% of recurrences occur on the same side as the first pneumothorax. Despite the documentation that pleural blebs occur bilaterally in many patients with PSP, the risk of contralateral pneumothorax is only 5% to 10%. Risk factors for recurrence that have been identified include greater height, lower weight, and smoking status.31
Death rarely occurs after PSP.32 In a study of spontaneous pneumothorax in which patient ages ranged from 15 to 34 years (most likely representing patients with PSP) the mortality rate was reported to be 0.09% for men and 0.06% for women.33
 SECONDARY SPONTANEOUS PNEUMOTHORAX
SECONDARY SPONTANEOUS PNEUMOTHORAX
Epidemiology
SSP is a more serious event than PSP because, by definition, the patient has underlying lung disease. A pneumothorax in a patient with already diminished pulmonary reserve can be life-threatening and requires more immediate medical attention and intervention.
The incidence of SSP is similar to that of PSP. An epidemiologic study in Olmsted County, Minnesota, documented an incidence of SSP of 6.3/100,000 year for males and 2/100,000 year for females.10 On average, patients with SSP are 15 to 20 years older than patients with PSP due largely to the older age of patients with emphysematous lung disease. The risk of recurrence for SSP is somewhat higher than for PSP and ranges from 40% to 80%, again, likely due to the underlying lung pathology.
Etiology
A variety of pulmonary diseases have been associated with spontaneous pneumothorax, but chronic obstructive pulmonary disease (COPD) is the most common. The Veterans Administration Cooperative Study on Pneumothorax noted that pneumothorax tended to occur in patients with moderately severe COPD, with a quarter of the participants having an FEV1 below 1 L and a mean FEV1/FVC ratio of 57%.34 Persistent bronchopleural fistula was also noted to be common in patients with obstructive lung disease, and 35% of patients had an air leak for more than 5 days.
Although airway diseases (COPD, cystic fibrosis [CF], and severe asthma) are the most common underlying lung disorders, a wide spectrum of pulmonary diseases has been associated with SSP (Table 78-1).35
In contrast to the low mortality rate associated with PSP, patients with SSP have a much higher risk of death. The VA Cooperative study reports an analysis of three papers that combined, reveal a mortality of 16% for SSP.34 Videm et al.36 showed that SSP increased the mortality of age-matched COPD patients by 3.5 times.
Cystic Fibrosis
Pneumothorax is a common and potentially serious complication of CF lung disease. It occurs more frequently in CF than in the general population and results in significant morbidity and mortality, typically occurring with more advanced disease. Approximately 3.5% of patients with CF will suffer this complication at some point in their lives. The majority occur in adulthood, with a median age of 21 years.37 Pneumothorax may also be a marker of poor prognosis with one study showing the median survival after the first spontaneous pneumothorax to be only 29.9 months.38
The pathophysiology of spontaneous pneumothorax in CF is likely related to chronic airway inflammation that eventually leads to structural changes with severe airflow obstruction; inflammation with poor clearance of thick, inspissated secretions; air trapping in the distal airways; and eventual rupture of the pleural surface with rising alveolar pressures.37
The risk of developing pneumothorax increases with age and with declining pulmonary function (FEV1). A recent study of pneumothorax in CF demonstrated that the risk of pneumothorax increased by 50% when FEV1 was <30% predicted.39 Other important risk factors for the development of pneumothorax include the presence of Pseudomonas aeruginosa, Burkholderia cepacia, or Aspergillus in the airways, and the use of Dornase alfa. The presence of these airway pathogens may increase inflammation and secretions leading to obstruction and the use of inhaled Dornase alfa may cause bronchospasm leading to acute decline in FEV1 with inhalation.40
CF-specific recommendations exist regarding the management of pneumothorax.41 A full discussion of these is beyond the scope of this chapter but several points regarding airway clearance in the setting of pneumothorax are of particular importance. With small pneumothoraces it is recommended that most airway clearance modalities could be continued, with the exception of those providing positive-pressure or percussive ventilation, which could further increase the size of pneumothorax. With large pneumothorax, it is recommended that airway clearance modalities should be held. Though the use of inhaled medications could provoke coughing, it is not recommended that these be held.
Recurrence of pneumothorax is more frequent in this population. An estimated failure rate of 37% with tube thoracostomy alone has been reported, but up to 70% of all patients have either an initial treatment failure, or an ipsilateral recurrence, ultimately needing more definitive treatment.42 Despite the recurrence rate being so high, the guidelines recommend reserving pleurodesis for large recurrent pneumothoraces.41 However, because the recurrence rate in CF is high, many CF centers still consider pleurodesis after the first episode. In the past, there was concern that pleurodesis would preclude patients from lung transplantation. However, recent studies have shown that pleurodesis does not add appreciably to complications during lung transplantation.43–45 Although center-dependent, pleurodesis is no longer considered a contraindication to transplantation.43
Catamenial Pneumothorax
Catamenial pneumothorax is a rare condition that occurs in women and is defined as recurrent pneumothoraces occurring within 72 hours of onset of menses. One study reported a mean of 3.17 episodes (range: 2–10) in a population of women prior to surgical treatment.46 This syndrome, by designation, occurs during the reproductive years, usually in the third or fourth decade of life; however, cases associated with hormone replacement have been reported.47 Though initially felt to be extremely rare, the condition is becoming increasingly recognized. A series from the Mayo Clinic found the condition in 5.6% of women with spontaneous pneumothorax over a 21-year period; however, more recent studies suggest the incidence of this disorder may actually be much higher.48,49
There is no single, definitive etiology for the development of catamenial pneumothorax. One theory suggests pleural and/or diaphragmatic endometriosis as the basis for this disorder; yet, only a fraction of women is found to have endometrial deposits at the time of surgical exploration. Alternative mechanisms that have been proposed include peritoneal air entering the thoracic cavity through diaphragmatic defects during menstruation or intercourse, endometrial implants in the terminal bronchioles causing obstruction, and increased circulating levels of prostaglandin F2α during menstruation resulting in bronchospasm and vasospasm.47 It is possible that various pathogenic mechanisms are at work.
The diagnosis, as mentioned above, is based on recurrent pneumothorax occurring within 72 hours of the onset of menses. Onset is typically heralded by chest pain and dyspnea. Over 90% of catamenial pneumothoraces affect the right hemithorax, but isolated left-side or bilateral pneumothoraces have been reported.
Given the unacceptably high recurrence rate of 50% to 100% with medical treatment alone, management with surgery followed by medical/hormonal therapy is typically indicated.47,49,50 Surgical treatment, which can be accomplished with video-assisted thoracoscopy, involves excision of endometrial implants, closure of any diaphragmatic defects, stapling of any blebs, and pleurodesis. Medical treatment is aimed at suppressing the ectopic endometrium and ovulation and at preventing further seeding. This is typically accomplished by using oral contraceptives, danazol, or gonadotropin-releasing hormone agonists. No controlled trials have examined the efficacy of one medication over another. If menses is not suppressed, there is a risk of recurrence, even after surgical treatment. Hysterectomy with bilateral oophorectomy will induce surgical menopause and has been employed to prevent pneumothorax.47
Pneumothorax in Acquired Immunodeficiency Syndrome
Patients with acquired immunodeficiency syndrome (AIDS) have a significantly increased risk of developing pneumothorax. Roughly 2% to 5% of AIDS patients experience spontaneous pneumothorax. In one study, pneumothorax complicated 1.2% of 599 HIV patient admissions over 3 years.51 Mortality was significantly higher (30.8%) in patients with pneumothorax than in those without (5.8%).
Pneumothorax in AIDS patients is associated with multiple infectious etiologies. Pneumocystis jiroveci, bacterial infections, Kaposi sarcoma, cytomegalovirus, pulmonary Cryptococcus, coccidiomycosis, and mycobacterial disease have all been associated with spontaneous pneumothoraces. Other risk factors for the development of pneumothorax in HIV include tobacco use, treatment with inhaled pentamidine, and the presence of a pneumatocele on chest radiograph.
Most studies have shown Pneumocystis jiroveci pneumonia (PCP) to be the primary etiology. However, a recent observational study of 105 HIV patients with spontaneous pneumothoraces found bacterial pneumonia to be the major underlying etiology (34.3%) followed by PCP (29.5%) and pulmonary tuberculosis (15.2%).52 In addition, the authors noted that bacterial pneumonia was a more common cause among drug users and those with CD4+ count over 200 cells/mL whereas PCP was a more common cause among patients with sexually transmitted HIV and CD4+ counts less than 200 cells/mL. Bilateral pneumothoraces were more commonly associated with PCP. Given the strong association with PCP, evaluation and treatment for PCP is recommended in any patient with AIDS who presents with an otherwise unexplained spontaneous pneumothorax.
The high frequency of pneumothoraces seen in patients with PCP is thought to be secondary to the presence of subpleural cysts and subpleural necrosis associated with this entity. Extensive tissue invasion within the alveolar interstitium is common in severe PCP and may result in subpleural necrosis. These cystic changes are thought to be due to repeated episodes of inflammation and cytotoxic effects of HIV on pulmonary macrophages with elastase production and necrosis of lung tissue.44,53 These lesions occur most frequently at the apices of the lungs and consist of necrotic alveoli filled with P. jiroveci organisms, macrophages, eosinophilic exudate, and fibrinous material.54 Histologic examination of patients who have recovered from PCP demonstrates subpleural blebs and bullae as well as pneumatoceles. Because of the necrotizing nature of the pneumonia, spontaneous pneumothorax from PCP is notoriously difficult to treat. Persistent air leaks often require tube thoracostomy for several weeks, and up to one-quarter of patients require surgical intervention. Some authors recommend early thoracoscopic therapy in good surgical candidates to avoid prolonged hospitalization.
The incidence of bilateral cystic disease in these patients is extremely high, and the incidence of contralateral pneumothorax was about 50% in one study.55 Therefore, if surgical intervention is planned, some authors recommend preoperative CT scan of the chest and median sternotomy in patients with significant bilateral disease to facilitate bilateral pleurodesis.
With the rise of AIDS, the frequency of pulmonary tuberculosis has increased. Pneumothorax has been reported in approximately 7% of HIV patients with pulmonary tuberculosis.56 All pneumothoraces associated with tuberculosis should be treated and often require prolonged periods of chest tube drainage. In cases of tuberculosis, it has been suggested that thoracotomy should not be considered until the patient has received antituberculous therapy for at least 6 weeks; however, objective evidence that this affects outcome is lacking.
 TRAUMATIC PNEUMOTHORAX
TRAUMATIC PNEUMOTHORAX
Trauma is the most common cause of pneumothorax. Patients who have either polytrauma or trauma to the thorax are at risk for pneumothorax. In one study of over 300 pneumothoraces, trauma was responsible for 56% of cases, the majority of which were iatrogenic.10 Noniatrogenic, traumatic pneumothorax can result from either penetrating or nonpenetrating chest injury. The diagnosis needs to be considered in any patient who is evaluated for significant trauma.
Penetrating chest trauma produces a pneumothorax by allowing air to enter the pleural cavity directly through the chest wall. In addition, if the visceral pleural is penetrated, air may leak from the tracheobronchial tree. If the continuity of the chest wall is disrupted, an open pneumothorax is produced. If the opening in the chest wall is larger than the diameter of the trachea (1.2–1.5 cm in an adult), air movement occurs through the pathway of least resistance, and air is preferentially inspired into the thoracic cavity through the open chest wound. Any open chest wound must be occluded to assure adequate ventilation of the patient.
Pneumothorax is also a frequent finding in patients with blunt trauma to the chest. The visceral pleura may be lacerated secondary to a rib fracture or dislocation; however, in almost one-half of patients with pneumothorax secondary to blunt trauma, there are no associated rib fractures. This is especially common with blunt trauma to the chest secondary to blast injuries and high-altitude falls into water. In such incidents, the abrupt increase in the pressure gradient between the alveolus and the adjacent bronchovascular sheath causes disruption of the alveolar membrane. Dissection of air through the interstitial space results in either pneumothorax or pneumomediastinum.
Occasionally, patients with traumatic pneumothorax have coexisting injuries of the tracheobronchial tree or of the esophagus. In a patient with a traumatic pneumothorax, fiberoptic bronchoscopy should be performed in the presence of hemoptysis or a persistent air leak. Eighty percent of injuries to the tracheobronchial tree are within 2.5 cm of the carina, most commonly on the right side at the membranous–cartilaginous interface. The main lobar bronchi and the cervical trachea are the next most common sites of injury.
Traumatic rupture of the esophagus usually produces a hydropneumothorax. Therefore, if a patient with a traumatic pneumothorax also has a pleural effusion, the possibility of esophageal rupture should be entertained. Almost all patients with perforation of the thoracic esophagus also have dysphagia and pneumomediastinum. An elevated pleural fluid amylase concentration is a reliable screening procedure for esophageal rupture. Once the diagnosis is suspected, contrast radiographic studies of the esophagus should be performed as soon as possible. Untreated, esophageal rupture results in mediastinitis and septic shock; therefore, a high index of suspicion is essential in making an early diagnosis.
Increasing utilization of invasive diagnostic and therapeutic interventions has significantly increased the rate of iatrogenic pneumothorax. These cases may result in considerable morbidity, prolonged hospitalization, and even death, for the affected patient.57 Iatrogenic pneumothorax can occur as a complication of multiple procedures, but the leading cause of iatrogenic pneumothorax is transthoracic needle aspiration followed by subclavian vein catheter insertion, thoracentesis, transbronchial biopsy, pleural biopsy, and positive-pressure ventilation.58 The incidence ranges between 20% to 40% with transthoracic needle aspiration; risk factors include COPD, depth of lesion within the lung, and lesions smaller than 2 cm.59–61 Pneumothorax may also occur with transbronchial needle aspiration, liver biopsy, intercostal nerve block, mediastinoscopy, and tracheostomy among others.
Central venous catheterization carries the second highest risk of iatrogenic pneumothorax. The reported risk ranges from 2% to 12% when using anatomic landmarks for guidance. Subclavian catheterization carries a higher risk than internal jugular catheterization. The increasing use of real-time sonographic guidance for central venous catheterization has significantly decreased this risk for both internal jugular and subclavian approaches.62,63 Similarly, the routine use of real-time ultrasound guidance for localization of needle puncture site during thoracentesis may decrease the risk of pneumothorax, which develops in approximately 5% of cases.64
Another cause of pneumothorax that is frequently overlooked is chest tube malfunction. Common causes of chest tube malfunction include inadequately securing the chest tube to the drainage system, failing to fill the U-tube manometer in the water seal chamber, failing to refill the water in the suction control chamber, and permitting intermittent disconnection of the system during diagnostic or therapeutic studies.
Mechanical ventilation is a frequent, potentially lethal cause of iatrogenic pneumothorax. The overall incidence of pneumothorax during mechanical ventilation ranges from 4% to 15%, but may be significantly higher in patients with underlying inflammatory diseases such as aspiration pneumonia.65 The incidence of pneumothorax is also increased during mechanical ventilation if patients have chronic pulmonary disease, are on increased amounts of positive end-expiratory pressure, or have right mainstem intubation.65 The incidence of pneumothorax has been reported to be 6.9% to 14% of patients with acute respiratory distress syndrome (ARDS).66,67 Another study reported a 48.8% incidence of pneumothorax in patients with severe ARDS requiring extracorporeal support.68
A pneumothorax should be suspected in any patient whose clinical status acutely decompensates on the ventilator. Bedside clues include a sudden increase in respiratory rate or appearance of patient-ventilator dyssynchrony. An increase in the peak and plateau pressures on the ventilator can be a sensitive indicator if the patient is on volume-cycled ventilation. The peak inspiratory pressure often rises suddenly as the lung compliance falls. If the patient is on pressure control ventilation, decreased tidal volumes will be a sign of pneumothorax.
Radiographs of critically ill, mechanically ventilated patients are frequently obtained only in the supine or semisupine position. In one study, supine and semierect radiographs were obtained in 88 critically ill patients with 112 cases of pneumothorax.69 The radiologist initially failed to detect the pneumothorax in 30% of the cases. Patients with extensive infiltrates, such as in the setting of ARDS, may have no suggestion of lung collapse on chest radiograph. In these patients, the only radiologic sign that may be evident is that of a deep sulcus on the side of the pneumothorax. This finding, as well as any increased lucency on a supine film, should raise suspicion of a pneumothorax and be evaluated by either erect or decubitus views, or ultrasound. If erect or decubitus films cannot be obtained or ultrasound is unavailable, CT scans of the chest may be necessary.
It should be noted that recipients of bilateral lung transplants and patients who have had a “clamshell” thoracotomy do not have an intact mediastinum. Because of this, the normally separate pleural spaces may be in direct communication with each other. Physicians performing procedures on these patients need to be aware that the patient can develop bilateral pneumothoraces because of this anomaly.70
CLINICAL FEATURES
The main symptoms with the development of a pneumothorax are chest pain and dyspnea, which occur in 95% of patients. The pain is usually acute, localized to the side of the pneumothorax, and typically pleuritic. Cough, hemoptysis, orthopnea, and Horner syndrome are uncommon manifestations of a pneumothorax.5,71 A small percentage of patients are asymptomatic or complain only of generalized malaise.
Spontaneous pneumothorax usually occurs at rest, and fewer than 10% occur during strenuous exercise. In PSP, both the dyspnea and chest pain may subsequently abate over the first 24 hours. This may explain why nearly half of patients have symptoms for 2 days before seeking medical attention and why 18% wait for more than a week.5 Most patients with SSP have more severe symptoms than patients with PSP, and dyspnea frequently seems out of proportion to the size of the pneumothorax.72
Small pneumothoraces (<20%) are usually not detectable on physical examination. In patients with obstructive lung disease, even larger pneumothoraces may be difficult to detect since decreased breath sounds and hyperresonance may already be present.73 On physical examination, vital signs are usually normal, with the exception of moderate tachycardia. Examination of the chest may reveal the affected side to be larger and move less during respiration. Tactile fremitus is absent, the percussion note is hyperresonant, and breath sounds are absent or reduced on the side with the pneumothorax. Hamman sign may be detected. This sign, also heard with pneumomediastinum, has been described as crunching or clicking noises synchronous with the heartbeat but influenced by respiration and body position. Severe tachycardia, with a heart rate above 140 beats a minute, hypotension, cyanosis, or tracheal deviation, suggests the possibility of a tension pneumothorax.
Arterial blood gases often show hypoxemia and perhaps hypocarbia from hyperventilation. Hypoxemia is usually mild in PSP when less than 25% of the lung is affected. When more than 25% of the lung is involved, pulmonary shunts occur more frequently and hypoxemia may be severe. In patients with SSP, pulmonary reserve is already diminished and life-threatening hypoxemia and hypercapnea may be present. In one study, the mean PaO2 was 48 mm Hg and the mean pCO2 was 58 mm Hg when patients with emphysema presented with a spontaneous pneumothorax.73
Patients with a left pneumothorax may show changes on EKG suggesting an anterolateral myocardial infarction. A rightward shift of the frontal QRS axis and clockwise rotation of the heart result in a diminution of precordial R-wave voltage, a decrease in the QRS amplitude, and precordial T-wave inversion.74 These electrocardiographic features differ from a transmural myocardial infarction because of the absence of ST-segment elevation or significant Q waves. An anterior subendocardial infarction may present with T-wave inversion but without the rightward shift in the frontal axis. The electrocardiographic changes with a left pneumothorax may normalize when the patient is in the upright or right lateral decubitus position.
RADIOGRAPHIC APPEARANCE
Below are considered the radiographic (plain radiography and CT) and ultrasound findings in pneumothorax.
 PLAIN RADIOGRAPHY
PLAIN RADIOGRAPHY
Classically, the diagnosis of pneumothorax is established by demonstrating the outer margin of the visceral pleura (and lung) separated from the parietal pleura (and chest wall) by a lucent space devoid of pulmonary vessels (Fig. 78-3) on chest radiograph, ultrasound, or CT scan. The pleural line may be difficult to detect with a small pneumothorax unless high-quality upright films are obtained. In erect patients, pleural gas collects over the apex, and the space between the lung and chest wall is most notable there. In the supine position, gas migrates along the broad ventral surface of the lung, making detection on a frontal radiograph difficult. In the supine position, the juxtacardiac area, the lateral chest wall, and the subpulmonic region are the best areas to search for evidence of pneumothorax. Lateral decubitus studies of the appropriate side may be helpful; however, several studies have shown that expiratory films have little or no advantage over upright inspiratory films in the diagnosis of pneumothorax.75–77 The most recent British Thoracic Society guidelines do not recommend the routine use of expiratory chest films in the evaluation of suspected pneumothorax.78
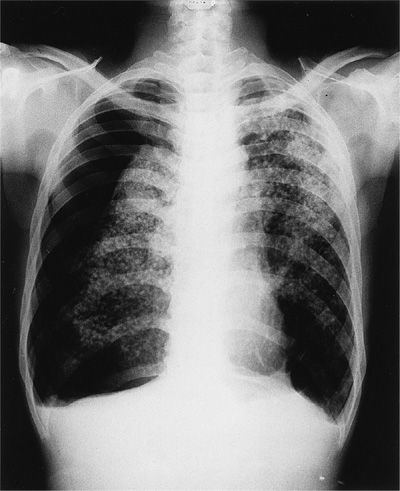
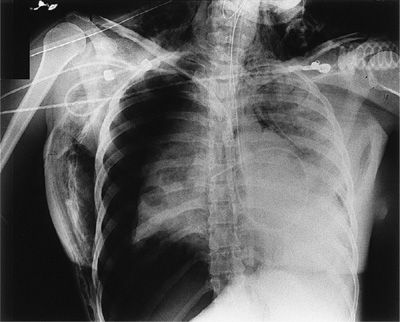
Figure 78-3 A patient with nodular silicosis and a spontaneous secondary pneumothorax. The visceral pleural line is clearly seen with the absence of vascular workings beyond the pleural line. There are cicatricial bullae in both bases.
It is very important to differentiate the pleural line of a pneumothorax on chest radiographs from that of a skinfold, clothing, tubing, or chest wall artifact. Careful inspection of the film may show that the artifact extends beyond the thorax, or that lung markings are visible beyond the apparent pleural line. In the absence of underlying lung disease, the pleural line of a pneumothorax usually parallels the shape of the chest wall. Artifactual densities generally do not parallel the course of the chest wall over their entire length. Avascular bullae or thin-walled cysts can be mistaken for a pneumothorax. The pleural line caused by a pneumothorax is usually bowed at its center toward the lateral chest wall. As opposed to a pneumothorax, the inner margins of bullae or cysts are, in general, concave rather than convex and do not exactly conform to the contour of the costophrenic sulcus. A pneumothorax with a pleural adhesion may also simulate bullae or lung cysts. An adhesion tends to form a straight line connecting the lung to the parietal pleura; bullae or cysts have rounded edges. Such features are not 100% specific, and if there is any doubt as to whether the patient has a bulla or cyst or a pneumothorax, a CT scan should be obtained as CT can usually differentiate the two.79 Pleural effusions may occur coincident with pneumothorax in up to 20% to 25% of cases. Hemopneumothorax occurs in 2% to 3% of cases of spontaneous pneumothorax. Bleeding is believed to represent rupture or tearing of vascular adhesions between the visceral and parietal pleura as the lung collapses.
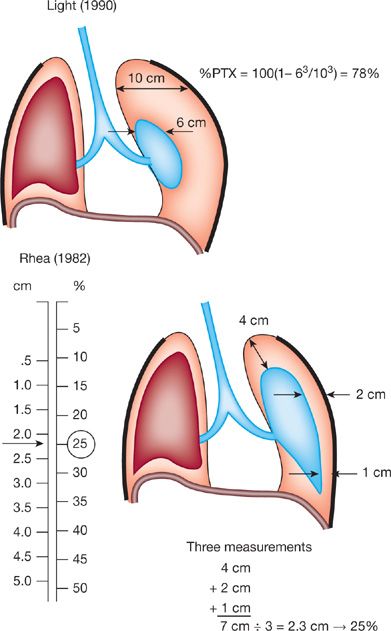
Quantification of the size of a pneumothorax on chest radiographs is helpful but the methods for quantifying lack uniformity and are by no means precise. Light suggested measuring the average diameters of the collapsed lung and of the affected hemithorax, then cubing these diameters to estimate the percentage of collapsed lung by the following equation: 100 × (1 – diameter of collapsed lung3/diameter of hemithorax3).71 For example, if the diameter of the collapsed lung is 6 cm and the diameter of the hemithorax is 10 cm, the collapsed lung is estimated by the formula 100 × (1 – 63/103). Thus, the estimated size of the pneumothorax is 78%.71 Rhea et al.80 proposed the use of a nomogram to calculate the size of the pneumothorax. With this method, the average intrapleural distance is calculated by measuring the interpleural distance at the apex and at the midpoints of both the upper and lower lungs. These three values are then averaged, and the number is reported on a nomogram, which gives an estimated size of the pneumothorax. An example of these calculations is shown in Figure 78-4.
Figure 78-4 Estimation of the size of the pneumothorax according to the method described by Light32 and Rhea et al.49 (Reproduced with permission from Beauchchamp. In: Pearson, ed. Textbook of Thoracic Surgery; 1995.)
The most common radiographic manifestations of tension pneumothorax are mediastinal shift, diaphragmatic depression, and rib cage expansion (Fig. 78-5). Any significant degree of displacement of the mediastinum from the midline position on maximum inspiration, or any depression of the diaphragm, should be taken as evidence of tension. The degree of lung collapse is an unreliable sign for or against the presence of a tension pneumothorax, since underlying lung disease may prevent collapse even in the presence of tension.
Figure 78-5 Right tension pneumothorax in a young patient with staphylococcal endocarditis and septic emboli. There is marked depression of the right hemidiaphragm, shift of the mediastinum, and subcutaneous emphysema. Note: The pulmonary artery catheter, endotracheal tube, and nasogastric tube (midchest) are all displaced to the left.
 LUNG ULTRASOUND
LUNG ULTRASOUND
Ultrasound has evolved to become an indispensable bedside tool for rapid diagnosis of pneumothorax. Since the early 1990s, our understanding of lung and pleural ultrasonography has continued to mature as new research has emerged and more clinicians have integrated point-of-care ultrasound into clinical practice. In an observational study of 260 consecutive patients admitted to an intensive care unit (ICU) with acute respiratory failure, experienced physicians performing lung ultrasound alone without additional clinical information were able to correctly diagnose the cause of acute respiratory failure in 90.5% of patients.81 An observational study of critically ill patients demonstrated that lung ultrasound facilitated diagnosis in 66% and guided management in 47% of patients.82 A similar more recent observational study in mechanically ventilated patients demonstrated that lung ultrasound changed management in 41% and revealed diagnoses not suspected by the treating physician in 21% of patients.83
Lung ultrasound is more sensitive than chest x-ray for detection of pneumothorax when using chest CT as the gold standard. Early studies by Lichtenstein et al. established superiority of lung ultrasound compared to chest x-ray to detect pneumothorax, with sensitivities and specificities ranging from 66% to 100% and 91% to 100%, respectively.84–87 Meta-analyses (13–21 studies) report a pooled sensitivity of 79% to 95% and specificity of 98% to 99% for lung ultrasound in detecting pneumothorax. Pooled sensitivity and specificity for chest radiography have been reported at 40% to 52% and 99% to 100%, respectively.88–90
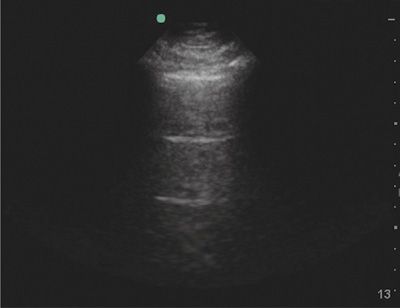
Normal lung and pleura are characterized by two ultrasound findings: lung sliding and A-lines. By conventional two-dimensional ultrasonography, normal visceral and parietal pleura appear as a bright white (hyperechoic) line that glistens with respirations as the visceral pleura slides back and forth against the parietal pleura (Video 78-1). A principal finding to diagnose pneumothorax is absence of lung sliding (Video 78-2). The presence of air in between the visceral and parietal pleura in pneumothorax prevents propagation of ultrasound waves beyond the parietal pleura, and the sliding, shimmering appearance created by apposed pleura is not seen.91,92 Presence of lung sliding definitively rules out pneumothorax with a negative predictive value of 100%84; however, absence of lung sliding is not specific to pneumothorax and can be seen with any cause of pleural symphysis (chemical pleurodesis, infectious/inflammatory adhesions, fibrosis) or lung volume loss (mainstem intubation, massive atelectasis, mucus plug, pneumonectomy, apnea).91 If lung sliding is absent, identification of the lung point, a specific sign of pneumothorax, can confirm the diagnosis. The lung point is the edge of a pneumothorax, or boundary between collapsed and aerated lung, and a lung point is 100% specific to rule in pneumothorax.87 The defining feature of lung point sign is visualization of aerated lung sliding into the field of view next to collapsed lung (Videos 78-3 and 78-4). The lung point can be found anteriorly when the pneumothorax is small, and laterally when it is large.92
Video 78-1 Normal lung sliding. This sagittal view using two-dimensional ultrasound shows normal lung sliding through an anterior chest interspace. Two ribs that cast shadows are seen on the left and right edges of the image. The pleural surface is seen below the ribs. The visceral and parietal pleura are apposed giving a shimmering, glistening appearance as the visceral pleura slides back and forth against the parietal pleura with respirations (See also Video 31-9). Access at www.fishmansonline.com
Video 78-2 Absence of lung sliding due to pneumothorax. Using two-dimensional ultrasound in a sagittal plane, the absence of lung sliding can be appreciated as the shimmering, glistening appearance of the pleura is not seen below the ribs (See also Video 31-10). Access at www.fishmansonline.com
Video 78-3 Lung point sign (lateral chest wall). The edge of a pneumothorax, or lung point, can be seen on the curvature of the lateral chest of a mechanically ventilated patient with a large, postprocedural pneumothorax. Aerated lung superiorly (left) slides into the field of view next to the collapsed, nonsliding portion of lung (right). Access at www.fishmansonline.com
Video 78-4 Lung point sign (anterior chest wall). The edge of a pneumothorax, or lung point, can be seen on the anterior, inferior chest wall of a patient with a small, spontaneous pneumothorax. Aerated lung slides in and out of the field of view next to the collapsed, nonsliding portion of lung (See also Video 31-11). Access at www.fishmansonline.com
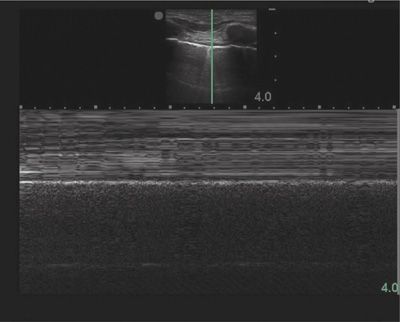
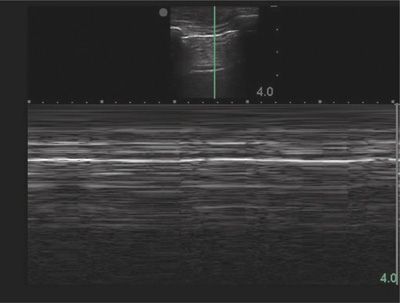
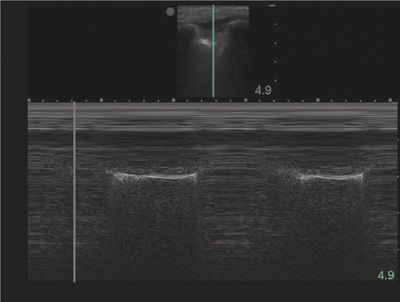
Evaluation of lung sliding should be confirmed using M-mode, or motion mode. M-mode displays movement of tissues over time. The normal M-mode pattern of lungs includes: horizontal, linear lines at the top, corresponding to the chest wall; a hyperechoic line corresponding to the pleura; and a granular pattern at the bottom corresponding to aerated lung parenchyma. The normal M-mode pattern is called “seashore” sign with the lung parenchyma representing the beach and the chest wall representing the ocean (Fig. 78-6). In pneumothorax, the granular pattern of lung parenchyma is lost, and horizontal, linear lines are seen throughout the display. The M-mode pattern of pneumothorax is often called “stratosphere” or “barcode” sign (Fig. 78-7).91 The lung point will display a distinct M-mode pattern with alternating segments of “seashore” and “barcode” patterns (Fig. 78-8).
Figure 78-6 Normal lung in M-mode. A two-dimensional ultrasound image at the top shows the sample line along which the movement of all tissues is recorded and displayed below. The chest wall creates a series of horizontal lines. Also noted are the hyperechoic line of the pleura and a granular pattern created by aerated lung.
Figure 78-7 Pneumothorax in M-mode. The separation of visceral and parietal pleura by air from a pneumothorax prevents ultrasound waves from reaching the visceral pleura resulting in loss of the granular pattern of normal lung at the bottom of the M-mode image. A series of horizontal lines is seen throughout the M-mode image and is often called “stratosphere sign” or “bar code sign.”
Figure 78-8 Lung point in M-mode. This M-mode image displays alternating patterns of normal lung and pneumothorax as normal, aerated lung slides over the area of collapsed lung.
A-lines are ultrasound image artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer. A-lines are seen as a series of equidistant horizontal white lines deep to the pleura (Fig. 78-9). A-lines are seen in normal lungs but also in pneumothorax, pulmonary embolism, COPD, or asthma. Therefore, presence of A-lines supports a diagnosis of pneumothorax but is not specific for pneumothorax. In contrast, the presence of B-lines, an artifact arising from the pleura, rules out pneumothorax with a negative predictive value of 100% (Video 78-5).86
Figure 78-9 A-lines. A-lines are a type of reverberation artifact seen as equidistant horizontal lines that are generated from reflections of the pleura. A-lines are seen in normal, aerated lungs, but are also seen in pneumothorax, pulmonary embolism, chronic obstructive pulmonary disease, and asthma.
Video 78-5 B-lines. B-lines are a type of reverberation artifact, also known as comet-tail artifacts, that arise from the pleural surface and extend vertically to the bottom of the screen as discrete, hyperechoic rays that slide with pleural movement. Presence of B-lines rules out pneumothorax (See also Video 31-12). Access at www.fishmansonline.com
A focused lung ultrasound examination to evaluate for pneumothorax can be performed rapidly—in less than 1 minute in one study by experienced clinicians.87 A phased array, linear array, or microconvex transducer is required. Specifically for evaluation of pneumothorax, a high-frequency (5–10 MHz), linear array transducer is preferred because of its higher resolution and sensitivity (82% vs. 76%) compared to convex array transducers.90 Air in the pleural space accumulates in the least dependent parts of the chest, which are anterior and inferior in supine patients. In a longitudinal plane with the transducer marker oriented cephalad, place the transducer in the midclavicular line, and scan each interspace sequentially evaluating for lung sliding. If absence of lung sliding is detected, move the transducer laterally in search of the lung point and to qualitatively assess the size of the pneumothorax. Use M-mode to confirm the presence or absence of lung sliding.92
 COMPUTED TOMOGRAPHY
COMPUTED TOMOGRAPHY
CT scans of the chest are being used with increasing frequency in patients with pneumothorax. CT scans may be necessary to diagnose pneumothorax in critically ill patients when upright or decubitus films are not possible. CT scans may also prove helpful in predicting the rate of recurrence in patients with spontaneous pneumothorax. One study demonstrated that patients who have larger or more numerous blebs on thoracic CT scans are more likely to have recurrence.93
Traumatic pneumothoraces, if large, can be detected both clinically and with chest radiography. However, a small, posttraumatic pneumothorax may be easily missed by both physical examination and chest radiograph. One prospective series revealed that 51% of trauma patients presented with an occult pneumothorax that was not seen on initial chest radiograph, but identified on CT imaging.94 In another large series looking at multiple trauma patients, 4.4% had a pneumothorax and 38.8% of these were detected only by CT scan.95 Early incorporation of a routine CT scan in all patients with chest trauma or multiple traumatic injuries may be required to successfully diagnose traumatic pneumothoraces.
Given the difficulties associated with diagnosing small pneumothoraces by plain chest radiograph, as well as the cost and delay of CT scans, thoracic ultrasound is quickly emerging as a valuable tool for the diagnosis of occult pneumothorax. The small size and portability of newer machines, as well as the advantage of immediate bedside interpretation, make ultrasound especially helpful in the management of critically ill and hemodynamically unstable patients. Most data has come from trauma literature where ultrasound has a more well-established role. Hand-held ultrasonography was shown to have better sensitivity (48.8 vs. 20.9%) and similar specificity compared with chest radiograph for detection of traumatic pneumothorax using CT as the gold standard.96 A review of ultrasound specifically in ICU patients concluded that ultrasound had a sensitivity of 95% and specificity of 94% compared to CT for the detection of pneumothorax.97 Ultrasound has the additional advantage of serving as a guide to direct the site of drainage.98
THERAPEUTIC OPTIONS
Therapy for pneumothorax is dependent on the size of the pneumothorax, symptomatology, and etiology. The basic tenets of therapy are to evacuate the space, achieve closure of the leak, and either prevent or reduce the risk of recurrence. A variety of treatment methods and adjuncts exist. The choice of therapy depends on many factors, including the clinical status of the patient, the cause of the pneumothorax, evidence for concomitant lung disease, prior history of pneumothorax, risk of recurrence, and, finally, the experience and preferred techniques of the physicians caring for the patient as well as the availability of specific therapeutic options.99 Major categories of treatment methods are listed below, followed by suggested guidelines for their application.
 OBSERVATION
OBSERVATION
Simple observation of the patient with a pneumothorax requires evidence that the air leak is sealed (i.e., that there is no further progression of the pneumothorax). This form of management is generally reserved for asymptomatic patients with a small (<20%) unilateral pneumothorax.
A suggested protocol is the performance of serial chest radiographs over the initial 24 hours to assess for further progression of the pneumothorax. Some have suggested that this approach could be performed safely on an outpatient basis with close observation and limited patient activity. This form of management is risky because complications may occur rapidly, with potential morbidity. In one study of observation, 5% mortality was reported owing to the development of tension pneumothorax from an unrecognized pleural leak.29 Inpatient monitoring during the initial phase of therapy also allows the use of adjunct measures such as supplemental oxygen, which increases the rate of absorption of pleural gas. Depending on the circumstances and level of patient compliance, continued follow-up may be done on an outpatient basis.
 ASPIRATION
ASPIRATION
Aspiration of pneumothorax has been advocated by some but has shown varied levels of success and there is poor consensus between guidelines as to which patients warrant this approach.100 The British Thoracic Society guidelines recommend simple aspiration as first-line therapy for all clinically stable patients with “large” first-time spontaneous pneumothorax.78 This is in contrast to the American College of Chest Physicians Delphi Consensus Statement on this issue, which advocates placement of a pleural catheter and drainage.101 The differences between selected guidelines are highlighted in Table 78-2.78,101–103