Pneumothorax
Alberto de Hoyos
Willard A. Fry
Classification
Pneumothorax is the collection of air in the pleural space—that is, between the lung and the chest wall. Pneumothoraces are classified as spontaneous and nonspontaneous, as shown in Table 58-1.248 Nonspontaneous pneumothoraces (NSPs) are either iatrogenic or due to trauma. Spontaneous pneumothorax (SPs) occurring in a patient without an immediately obvious underlying lung disease is termed primary spontaneous pneumo- thorax (PSP). Secondary spontaneous pneumothorax (SSP) occurs in the presence of an underlying lung condition, such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), or infection with Pneumocystis jiroveci (formerly Pneumocystis carinii) pneumonia (PJP). PSP, which implies absence of underlying lung disease, is misleading, since most patients undergoing surgical intervention for the prevention of recurrence have identifiable emphysema-like changes (ELCs) such as blebs or bullae.255
Pneumothorax is classified further as open or closed, depending on the presence or absence of air leak at the time of presentation and intervention. This distinction is fundamental in selecting the appropriate initial therapy, as described below.
Consensus Statement and Guidelines for the Management of Spontaneous Pneumothorax
Four sets of guidelines have been published to provide the clinician with recommendations for the evaluation and management of adult patients with primary and secondary spontaneous pneumothorax.26,69,112,244 The American College of Chest Physicians (ACCP) Delphi Consensus Statement provides expert recommendations in areas where there is insufficient clinical evidence based on literature review from 1967 to 1999. The level of agreement among the 30 participating investigators for each recommendation ranges from no consensus to perfect consensus. The British Thoracic Society (BTS) makes recommendations based on level of evidence published in the literature (grades A to C). The Belgian and Australian guidelines provide similar recommendations. Despite publication of these guidelines, management of pneumothorax continues to be characterized by empiricism rather than being evidence-based. In addition, there is substantial lack of awareness and implementation of these guidelines by practicing physicians.143 The approaches and recommendations outlined by the ACCP (level of consensus) and BTS (grade of evidence) are complementary, with only rare areas of divergence as outlined below.
Incidence, Recurrence Rates, and Mortality
More than 20,000 new cases of SP occur each year in the United States at a cost of more than $130 million.194 The incidence of PSP and SSP in the United States is roughly equal: 18 to 28 per 100,000 men and 1.2 to 6 per 100,000 women per year.194 PSP typically occurs in tall, thin males between the ages of 12 and 30 years; it rarely occurs after the age of 40.236 Cigarette smoking increases the risk of PSP in men by as much as a factor of 20 in a dose-dependent fashion.29,102 The incidence of SSP is 6.3 and 2 per 100,000 per year for men and women respectively.194 The major causes of SSP are depicted in Table 58-2. The peak incidence occurs later in life (60–65 years), paralleling the incidence of chronic lung disease in the general population. Recurrence rates for PSP and SSP vary widely in the literature, partly owing to differences in duration of follow-up and choice of treatment.176,181,194,278 The largest randomized controlled trial assessing recurrence prevention by chest tube–directed tetracycline versus chest tube alone noted recurrence rates of 31.8% in PSP and 43% in SSP patients not undergoing treatment to prevent recurrence.178 In a compilation of 11 studies of PSP in which patients were treated by observation, needle aspiration, or chest tube without intervention to prevent recurrence, the average rate of recurrence was 30%, with a range of 16% to 52%.255 Recurrence after a second episode is as high as 67%. Most recurrences in PSP and SSP patients not being treated to prevent recurrence are seen in the first 6 months to 2 years of follow-up.112,181 Independent risk factors for recurrent pneumothorax include the presence of pulmonary fibrosis, asthenic habitus, younger age, age ≥60, smoking, and increased height-to-weight ratio.105,112,181 In contrast, evidence of ELCs on computed tomography (CT) or video-assisted thoracoscopic surgery (VATS) is not predictive of recurrence and such evidence by itself should not form the basis of decisions regarding the prevention of recurrence.189,200,209,215,225,260,262,285 In other studies, however, CT demonstrating ELCs in the contralateral lung correlated with a slightly increased risk of contralateral recurrence (10%–15%).124,260 Recurrence prevention is a more
important consideration in patients with SSP, given their intrinsically limited pulmonary reserve and higher pneumothorax-related mortality rate (3.5-fold).23,248 Death rarely occurs in the setting of PSP (0.09% for men and 0.06% for women) but is more common in patients with SSP (1%–3%) as a result of compromise of pulmonary reserve from the underlying lung disease.79,98,106,178,194,278
important consideration in patients with SSP, given their intrinsically limited pulmonary reserve and higher pneumothorax-related mortality rate (3.5-fold).23,248 Death rarely occurs in the setting of PSP (0.09% for men and 0.06% for women) but is more common in patients with SSP (1%–3%) as a result of compromise of pulmonary reserve from the underlying lung disease.79,98,106,178,194,278
Table 58-1 Classification of Pneumothorax | |
|---|---|
|
Etiology and Pathophysiology
Primary Spontaneous Pneumothorax
The exact pathophysiology of PSP remains unclear regarding the exact site of the air leaks, its underlying disease process, and its precipitating causes. The most common cause of PSP is believed to be the rupture of an apical subpleural bleb, but the exact precipitating event remains unknown22,210,211 (Fig. 58-1). A strong etiologic association has been proposed between ELCs and the occurrence of PSP.22 Up to 81% of patients with PSP have ELCs on CT scans, as compared with 0% to 20% of controls matched for age and smoking status.30,172,200,256,285 CT scans reveal that ELCs are often bilateral and located predominantly in the apical segments of the upper and lower lobes.124,209,260 ELCs in the contralateral lung are found in as many as 79% to 93% of patients managed by sternotomy.255 The exact mechanism of bulla formation remains speculative. A plausible explanation is that degradation of elastic fibers in the lung is induced by the smoking-related influx of neutrophils and macrophages, causing an imbalance in the protease–antiprotease and oxidant–antioxidant systems.255 Some authors have postulated a difference in alveolar pressure in the upright human between the base and apex of the lung. The ectomorphic physique often noted in PSP patients, characterized by the rapid increase in vertical size of the thorax during early childhood and adolescence, may affect intrathoracic pressure and drive subpleural cyst formation (ELC formation).95 Familial patterns of inheritance (autosomal dominant, X-linked recessive, FBN1 gene mutation, HLA haplotypes, and autosomal recessive), although uncommon, continue to be reported in patients with PS.1,24,41,150,202,293 Respiratory bronchiolitis is found in more than 80% of smokers operated on for PSP when blebs, bullae, or areas of air leaks are surgically resected and may contribute to the pathogenesis of ELCs.63
Table 58-2 Causes of Secondary Spontaneous Pneumothorax | |
|---|---|
|
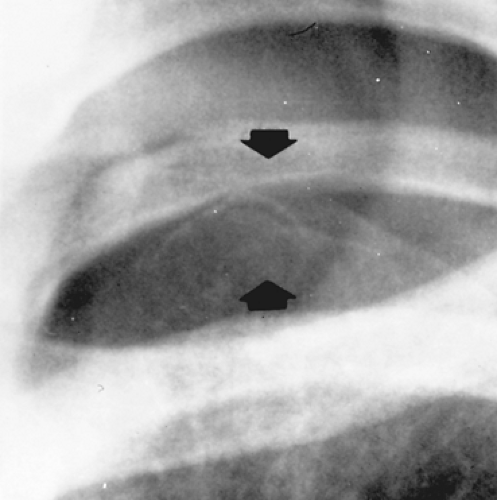 Figure 58-1. Chest radiograph showing apical subpleural blebs (arrows) in a male adolescent with a recurrent spontaneous pneumothorax. |
Despite the strong evidence that ELCs cause PSP, they may not be the sole cause, as suggested by the fact that air leak may occur in the absence of ELCs or in areas distant from ruptured ELCs.210,211,212,255 This observation has led to the concept of “pleural porosity” as a factor in PSP, as demonstrated by fluorescein-enhanced autofluorescence thoracoscopy.211,217 This debate is not purely academic, since optimal prevention of recurrence must address all potential etiologic issues effectively. In addition,
there is strong argument that pleurodesis and not bleb excision alone is the cornerstone of preventing recurrence.24,211
there is strong argument that pleurodesis and not bleb excision alone is the cornerstone of preventing recurrence.24,211
Secondary Spontaneous Pneumothorax
The causes of secondary pneumothorax are listed in Table 58-2. Airway diseases, including COPD, CF, and asthma, lead the list of causes of SSP, followed by infections such as PJP, tuberculosis, and necrotizing pneumonia. Less common causes include fibrotic lung disorders such as sarcoidosis, Langerhans cell granulomatosis, and lymphangiomyomatosis. Connective tissue disorders, cancer, and thoracic endometriosis syndrome (catamenial pneumothorax) should also be considered as causes of SSP. The most common sarcomas contributing to pneumo- thorax are osteosarcoma and synovial sarcomas.195 On occasion, a pneumothorax may be secondary to esophageal perforation in the absence of significant esophageal symptoms and may be overlooked. A high index of suspicion is necessary to avoid delayed diagnosis and unfavorable outcome.
Spontaneous pneumothorax occurring in patients with established severe COPD or bullous disease is troublesome. These patients tolerate even small degrees of collapse poorly, and they should be treated with chest drains rather than by observation (Table 58-3).26,112 This group of patients is discussed in detail in Chapters 87 and 88. Spontaneous pneumothorax complicating CF is associated with severe disease and can be life-threatening.112 Median survival after pneumothorax in patients with CF is 30 months; its occurrence reflects the severity of the underlying disease rather than being an independent risk factor. Contralateral pneumothoraces occur in up to 40% of patients.94,220,264
Catamenial pneumothorax (CP) is defined as a recurrent pneumothorax occurring within 72 hours of the onset of menstruation, although somewhat different time frames may be possible, such as the immediate premenstrual period and the ovulatory phase.8 CP is part of the thoracic endometriosis syndrome (TES), which also includes catamenial hemothorax, catamenial hemoptysis, and lung nodules. TES refers to the clinical and radiologic manifestations associated with the growth of endometrial glands and stroma in the lungs, pleural surfaces, diaphragm, and rarely the tracheobronchial tree. Unlike pelvic endometriosis, TES is a rare condition. The etiologic mechanisms of this syndrome are not well understood, and different theories have been proposed.8 None of the theories can explain all the clinical manifestations, and the disease probably has a multifactorial etiology.8 Of interest, pulmonary endometriosis is characterized by bilateral involvement, whereas pleural and diaphragmatic lesions and the occurrence of pneumothorax is mostly unilateral and right-sided in almost all cases.140,153 The mean age at presentation is 35 years, with a range from 15 to 54 years. Catamenial pneumothorax is the most common clinical presentation of TES and may be responsible for a larger proportion of pneumothoraces in young women than previously appreciated.6,7,140,152,153,230,233 Diaphragmatic abnormalities, including fenestrations and endometrial implants, are common and may play a role in the pathogenesis.140 Endometriosis in the pleural or diaphragmatic surfaces is characterized by blue–brown implants or “chocolate cysts.” Diaphragmatic abnormalities are the most commonly described anormality, with the tendinous portion being the most frequently involved part of the diaphragm. The perforations are generally a few millimeters wide, although larger defects, through which the liver is easily identified, are not infrequent. Associated endometrial deposits range from few millimeters to more than a centimeter in size. The clinical presentation of CP includes cough, chest pain, and shortness of breath. On occasion, catamenial hemothorax may present in association with CP, but it is uncommon.140
Table 58-3 Differences Between Primary and Secondary Pneumothorax | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Pneumothorax in association with pregnancy has been reported in approximately 1 of every 10,000 deliveries.158 The mean gestational age for a first occurrence is 26 weeks, but there is wide variation; 33% occurr at or near term. The recurrence rate is 44%, with the majority of these occurring during the same pregnancy or postpartum. In a collective review of 41 patients, universally favorable outcomes for both mother and baby were reported.90,158 Pneumothorax during pregnancy in association with a pulmonary synovial sarcoma has also been described.90 Spontaneous pneumothorax may occur as a rare manifestation of lung cancer, contributing to only 0.03% to 0.05% of cases of spontaneous pneumothorax.
There is now strong evidence that changes in atmospheric pressure are related to the development of spontaneous pneumothorax events, as reflected by the appearance of
microepidemics during thunderstorms and temperature fluctuations, particularly warm, dry weather and the passing of a cold front.5,34
microepidemics during thunderstorms and temperature fluctuations, particularly warm, dry weather and the passing of a cold front.5,34
The incidence of iatrogenic pneumothorax is high, outnumbering PSP in a large review series.72 The five leading causes include transthoracic needle aspiration (24%), subclavian vessel puncture (22%), thoracentesis (22%), pleural biopsy (8%), and mechanical ventilation (7%). The two primary risk factors in patients undergoing transthoracic needle biopsy include depth of the lesion (subpleural lesion) and COPD.249,295,296 Other risk factors include size of the lesion (<2 cm), transgression of several pleural planes, size of the needle (18 gauge), and wider trajectory angle of the needle.99,295,296 The use of ultrasound guidance for percutaneous central vascular cannulation is recommended to reduce the risk of pneumothorax.100 The treatment of iatrogenic pneumothorax tends to be simple as there is less likelihood of recurrence. With the exception of barotrauma, the majority of iatrogenic pneumothoraces are small and “closed” (no ongoing air leak) and resolve with observation alone. Treatment, if required (4%–20%), should be by simple aspiration or small-bore drains (<14 Fr).
Pneumothorax has also been described folllowing radiofrequency ablation of lung tumors, acupuncture, illicit drug use, gastrointestinal endoscopy, and during intubation for anesthesia.74,265,280,288,289,299 Barotrauma pneumothorax is defined as that occurring in a patient receiving positive-pressure ventilation. Ventilation-induced lung injury can result in rapid deterioration and result in a tension pneumothorax, a life-threatening condition. This topic is discussed in more detail in Chapter 74. Traumatic pneumothorax resulting from either blunt or penetrating chest trauma is dealt with in Chapter 73.
Clinical Presentation, Physical Examination, and Diagnosis
In the absence of an associated disorder, PSP is rarely seen before puberty. It is more common in men than in women by a ratio of 6:1, and it is more common in smokers than in nonsmokers.25,65,196,248,255 The typical patient with SP is a young, tall, thin male in late adolescence or early adulthood who experiences the sudden onset of pleuritic chest pain and dyspnea at rest or during normal activities. This clinical picture contrasts with spontaneous pneumomediastinum, which is commonly associated with strenuous exertion, instrumentation of the upper aerodigestive tract, or other associated conditions such as asthma. Many patients with PSP do not seek medical advice for several days, 46% waiting more than 2 days before presentation despite symptoms. Patients with PSP are on average taller (2 inches) and thinner (10 lb underweight) compared with reference averages for height and weight.291 Tachycardia is the most common physical finding, and the larger the pneumothorax, the more likely additional findings may be present, such as decreased movement of the chest wall, hyperresonance to percussion, diminished fremitus, and decreased or absent breath sounds on the affected side. In instances of mild collapse, physical findings can be misleadingly normal and a chest radiograph may confirm the suspected diagnosis. Symptoms usually resolve within 24 hours, even if the pneumothorax remains untreated and does not resolve.
In contrast to the benign clinical course of PSP, SSP is a potentially life-threatening event, because patients have associated lung disease and limited cardiopulmonary reserve. In patients with SSP, dyspnea may be severe and out of proportion to the size of the pneumothorax, and hypoxemia, hypercarbia, and hypotension may be the dominant findings.248 Symptoms do not resolve spontaneously in patients with SSP. The probability of pneumothorax increases as COPD worsens, and patients with a FEV1 of less than 1 L or a ratio of FEV1 to FVC of less than 40% are at greatest risk. The physical findings are often subtle and may be masked by the underlying lung disease. Tension pneumothorax (TPT) is an uncommon complication of SP, with a malignant course leading to death if untreated.169,247 TPT is more commonly found in trauma units, emergency departments, and intensive care units.169 The clinical presentation of TPT is characterized by chest pain, tachycardia, severe dyspnea, diaphoresis, hypotension, and pallor. Symptoms result from mediastinal shifting, reduced preload and cardiac output, and intense stimulation of the sympathetic nervous system. TPT is described in more detail in the discussion of complications, below.
Imaging of Pneumothorax
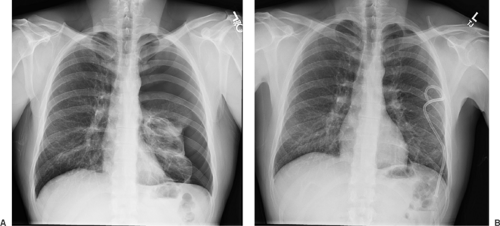
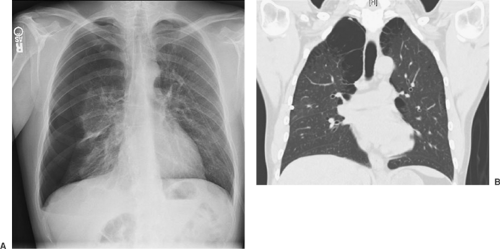
The diagnosis of PSP is suggested by the patient’s history and confirmed by the identification of a thin, visceral pleural line (<1 mm in width) that is found to be displaced from the chest wall on a posteroanterior chest radiograph obtained with the patient in an upright position (Fig. 58-2).23,196,248,255 A radiograph obtained during exhalation may help identify a small apical pneumothorax; however, the routine use of this technique does not improve the diagnostic yield and is not recommended in the BTS guidelines (grade B).112 In the presence of adhesions between the lung and the chest wall, the lung collapses unevenly or partially, giving the appearance of a loculated pneumothorax. In patients suspected of having SSP secondary to COPD, a giant bulla may appear as a pneumothorax and should be clearly distinguished before proceeding with any intervention (Fig. 58-3). A clue to the presence of pneumothorax is a visceral pleural line that runs parallel to the chest wall; bullous lesions that abut the chest wall have a concave appearance.23,248 If the distinction is not clear, a CT scan of the chest should be performed to differentiate between the two conditions, because only pneumothorax should be treated with a drainage procedure. On CT examination, the presence of thin strands of tissue within the airspace confirms the diagnosis of bulla. Additional findings in pneumothorax may include mediastinal and subcutaneous emphysema or a small pleural effusion. On occasion a large effusion may represent a hemothorax secondary to a torn vascular adhesion. Skin folds are occasionally misinterpreted as pneumothorax. The skin fold artifact, however, has a denser appearance just under the “line,” which is the opposite of a pneumothorax, in which the lung is lucent. Although CT of the chest gives an excellent evaluation of the pneumo- thorax, its routine use and cost-effectiveness remain in question (Fig. 58-4). CT is particularly effective in demonstrating ELCs.
Unfortunately, no absolute correlation has been found between the number or size of ELCs with an absolute risk of pneumothorax recurrence.24 Despite the lack of a clear recurrence predictive value of CT, some have used the presence of blebs >5 mm by high-resolution CT to select patients with an initial PSP for early operative intervention, but this approach remains to be proven.147 The ACCP does not recommend routine CT for first-time PSP or SSP. The BTS recommends CT in differentiating pneumothorax from complex bullous disease, when aberrant tube placement is suspected, and when the plain radiograph is obscured by surgical emphysema (grade C). Further study is needed before CT scanning can be recommended in the management of selected patients with spontaneous pneumothorax. Small fluid collections are frequently encountered if the pneumothorax lasts longer than 24 hours. The fluid is usually clear, and it is not necessary to analyze it. Large effusions are often bloody and suggest a torn vascular adhesion.
Unfortunately, no absolute correlation has been found between the number or size of ELCs with an absolute risk of pneumothorax recurrence.24 Despite the lack of a clear recurrence predictive value of CT, some have used the presence of blebs >5 mm by high-resolution CT to select patients with an initial PSP for early operative intervention, but this approach remains to be proven.147 The ACCP does not recommend routine CT for first-time PSP or SSP. The BTS recommends CT in differentiating pneumothorax from complex bullous disease, when aberrant tube placement is suspected, and when the plain radiograph is obscured by surgical emphysema (grade C). Further study is needed before CT scanning can be recommended in the management of selected patients with spontaneous pneumothorax. Small fluid collections are frequently encountered if the pneumothorax lasts longer than 24 hours. The fluid is usually clear, and it is not necessary to analyze it. Large effusions are often bloody and suggest a torn vascular adhesion.
Size Estimation of the Pneumothorax
It is commonly held that size of pneumothorax is an important determinant of therapy.26,112,248,255 However, best-guess
estimation of pneumothorax size is inaccurate and inconsistent between observers. Estimation of the size of a pneumothorax on plain chest radiographs as percent of collapsed lung has been reported by Rhea,245 Collins,60 and Light.175 The plain posteroanterior chest radiograph is, however, a poor method for quantifying the size of a pneumothorax, usually underestimating its size.87 This is largely because its two-dimensional image is a poor representation of what is really a space of complex shape. Several alternative methods have been proposed. Although estimation of size by such formulas may be accurate in PSP, abnormal collapse of the lung in SSP may preclude accurate calculations. The Light index suggests that the volume of a pneumothorax can be calculated in terms of percentage as 100 – (average diameter of the lung3/average diameter of hemithorax3) × 100. The diameters are measured at the hila. There are conflicting data regarding the validation of this method.116,213 The Rhea method uses the average of the interpleural distances measured in centimeters at the apex, midpoint of the upper half of the lung, and midpoint of the lower half of the lung on an erect chest radiograph to estimate pneumothorax size in percent using a nomogram. This method is accurate for smaller pneumothoraces but prone to underestimate larger ones.145 More accurate CT estimates of pneumothorax highlight the poor prediction of chest radiograph–calculated pneumothorax size. Collins60 derived a formula based on these data using interpleural distances on an erect chest radiograph to estimate percent pneumothorax as size % = 4.2 + 4.7 × (sum of interpleural distances in centimeters at apex, midpoint of upper half of collapsed lung, and midpoint of lower half of collapsed lung). This method, although more robust in development, has yet to be validated. This calculation is cumbersome for everyday use; however, practical cutoffs approximating 20% and 60% hemithorax volume can be derived with a total of the sum of the interpleural distances of 3.5 cm estimating a 20% pneumothorax and a total of 12 cm estimating a 60% pneumothorax.145 An automated format for calculating the size of a pneumothorax is available on the Web: http://www.chestx-ray.com/calculator/PTX.html. All these methods are cumbersome and generally used only as research tools. The ACCP and BTS guidelines use a combination of pneumothorax size estimation and the patient’s clinical status to direct management.26,112 The ACCP considers a small pneumothorax to be “less than 3 cm in collapse from apex to copola” (46% by the Collins formula if the lung collapses uniformly = 9 cm), while the BTS notes small to be “a rim of air less than 2 cm in collapse between the lung and the chest wall” (32% by the Collins formula = 6 cm). Neither guideline uses a percentage of lung collapse to direct therapy. A simplified method classifies a large pneumothorax as complete separation of the lung from the chest wall and a small pneumothorax as partial separation.
estimation of pneumothorax size is inaccurate and inconsistent between observers. Estimation of the size of a pneumothorax on plain chest radiographs as percent of collapsed lung has been reported by Rhea,245 Collins,60 and Light.175 The plain posteroanterior chest radiograph is, however, a poor method for quantifying the size of a pneumothorax, usually underestimating its size.87 This is largely because its two-dimensional image is a poor representation of what is really a space of complex shape. Several alternative methods have been proposed. Although estimation of size by such formulas may be accurate in PSP, abnormal collapse of the lung in SSP may preclude accurate calculations. The Light index suggests that the volume of a pneumothorax can be calculated in terms of percentage as 100 – (average diameter of the lung3/average diameter of hemithorax3) × 100. The diameters are measured at the hila. There are conflicting data regarding the validation of this method.116,213 The Rhea method uses the average of the interpleural distances measured in centimeters at the apex, midpoint of the upper half of the lung, and midpoint of the lower half of the lung on an erect chest radiograph to estimate pneumothorax size in percent using a nomogram. This method is accurate for smaller pneumothoraces but prone to underestimate larger ones.145 More accurate CT estimates of pneumothorax highlight the poor prediction of chest radiograph–calculated pneumothorax size. Collins60 derived a formula based on these data using interpleural distances on an erect chest radiograph to estimate percent pneumothorax as size % = 4.2 + 4.7 × (sum of interpleural distances in centimeters at apex, midpoint of upper half of collapsed lung, and midpoint of lower half of collapsed lung). This method, although more robust in development, has yet to be validated. This calculation is cumbersome for everyday use; however, practical cutoffs approximating 20% and 60% hemithorax volume can be derived with a total of the sum of the interpleural distances of 3.5 cm estimating a 20% pneumothorax and a total of 12 cm estimating a 60% pneumothorax.145 An automated format for calculating the size of a pneumothorax is available on the Web: http://www.chestx-ray.com/calculator/PTX.html. All these methods are cumbersome and generally used only as research tools. The ACCP and BTS guidelines use a combination of pneumothorax size estimation and the patient’s clinical status to direct management.26,112 The ACCP considers a small pneumothorax to be “less than 3 cm in collapse from apex to copola” (46% by the Collins formula if the lung collapses uniformly = 9 cm), while the BTS notes small to be “a rim of air less than 2 cm in collapse between the lung and the chest wall” (32% by the Collins formula = 6 cm). Neither guideline uses a percentage of lung collapse to direct therapy. A simplified method classifies a large pneumothorax as complete separation of the lung from the chest wall and a small pneumothorax as partial separation.
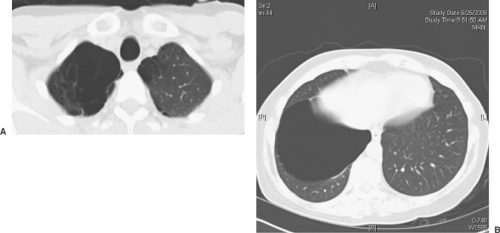 Figure 58-3. A: CT of the chest demonstrating a large bulla. Note lung tissue strands across the bulla. B: CT of the chest demonstrating right-lower-lobe bulla. |
Complications of Spontaneous Pneumothorax
Persistent Air Leak
Persistent air leak is present when there is evidence of a leak 48 hours after chest tube insertion. Although rare in PSP, it is more common in patients with SSP with obstructive or fibrotic lung disease. If there is incomplete lung reexpansion, a second tube may be required, although the ACCP recommends against placement of an additional chest tube to attempt to seal persistent air leaks (very good consensus). After 4 days of drainage, surgery should be considered to close the air leak and perform a pleurodesis to prevent recurrence (very good consensus).23,25,26,112,248,275 Selected patients with SSP at high risk for surgery can be discharged with a Heimlich valve and observed for a longer period of time.58,71,281
Pneumomediastinum
Pneumomediastinum occurs owing to the propagation of air from a site of injury in the lung parenchyma into the mediastinum along the bronchovascular bundles.248 Like subcutaneous emphysema, it is of no clinical consequence, but if it is progressive, it may require application of suction or additional chest drains for resolution.
Hemopneumothorax
Hemopneumothorax occurs in 5% to 10% of cases and is more common in men than in women.122 The hemorrhage occurs from a torn vascular adhesion or a vascular bleb that fails to tamponade. The mean blood loss is approximately 1,012 mL, and one-third of patients present with signs of hypovolemic shock.122 Although lung reexpansion may help tamponade the bleeding site, early surgical intervention is warranted to stop the bleeding, evacuate the clotted hemothorax, resect the site of air leak, and proceed with pleurectomy or pleurodesis.108,121 VATS has emerged as the preferred treatment option, resulting in a short hospital stay, infrequent need for transfusion, and no recurrences.51,129,182
Tension Pneumothorax
Tension pneumothorax (TPT) is thought to result from the operation of a one-way valve system, allowing air into the pleural space during inspiration but not allowing it out during exhalation.169 Although there is widespread misunderstanding that the presence of mediastinal shift plus depression and flattening of the diaphragm constitute TPT and a medical emergency, TPT is a clinical diagnosis defined as “any pneumothorax with cardiorespiratory compromise or collapse.” The diagnosis of tension is based on clinical disturbances and not on radiographic findings alone. In simple pneumothorax, mediastinal shift may occur owing to the loss of negative pressure in the hemithorax, leading to imbalance and contralateral shift of the mediastinum; by itself it does not constitute tension.245 TPT occurs rarely in patients with PSP but is more common in patients with SSP or in victims of trauma or patients who are intubated (barotrauma).43,59,117,141,169,266 Patients with TPT show signs of respiratory distress, tachycardia, anxiety, hypotension, cyanosis, and a hyperexpanded hemithorax that does not move with respiration and tracheal deviation. It is more a visual than auscultatory diagnosis (absence of breath sounds) and requires emergency decompression with a needle or chest tube without confirmatory imaging168,286,298 If a chest radiograph is performed, abnormalities include tracheal deviation, increased thoracic volume, increased rib separation, contralateral lung compression, and a deep sulcus sign. TPT should be suspected in any intubated or postoperative patient developing hypotension and is often missed in the intensive care unit (ICU) setting.54,93,234 The presence of a chest tube does not preclude its development, as the chest tube may be within the fissure or the lung and surrounded and occluded by lung parenchyma.18,36,192 TPT can be rapidly progressive and life-threatening in the ICU, particularly in mechanically ventilated patients.169 Treatment consists in needle decompression followed by tube thoracostomy. The needle should be at least 7 cm long and inserted in the midclavicular line at the second intercostal space. A standard 14-gauge (4.5-cm) cannula may not be long enough to penetrate the parietal pleura. A syringe filled with sterile saline attached to the cannula may help confirm pleural penetration. If the patient is breathing spontaneously and is suspected of having an enlarging pneumothorax, a chest radiograph should be obtained as quickly as possible to document its presence and avoid placing a needle to “rule out” a TPT. Morbidity associated with needle decompression may be significant and includes pneumothorax, cardiac tamponade, hemorrhage, and lung laceration.169 Some cases of TPT do not respond to needle decompression and require emergent chest tube placement for restoration of hemodynamics.43,139 TPT can also occur in a mechanically ventilated patient whose pneumothorax is treated with a small-bore chest tube (14 Fr).27,292 Other reported causes of TPT include acupuncture, colonoscopy, and autologous blood patch pleurodesis for persistent air leak.131,134,290
Recurrence
Recurrence is the most frequent complication of PSP and is seen in 30% of cases.248,255 Recurrence is defined as a pneumothorax that appears in the ipsilateral side >7 days after a pneumothorax has resolved. Most recurrences are seen during the first 6 months to 2 years of follow-up. A contralateral pneumothorax may occur in up to 25% to 40% of cases. After a second PSP, the risk of a subsequent event increases to >60%.
Treatment
The management of pneumothorax centers on evacuating air from the pleural space and preventing recurrences.26,112,248,255 The best treatment remains unknown and continues to be debated. Treatment options are listed in Table 58-4. Management options range from observation, simple aspiration, small-bore catheter insertion, and tube thoracostomy to VATS and thoracotomy with bleb excision and pleurodesis.26,112,248,255 Selection of an approach depends on the size of the pneumothorax, severity of symptoms, whether there is an open (persistent air leak) or closed (no air leak) pneumothorax, and whether the pneumo- thorax is primary, secondary, or recurrent. Thoracic surgeons, pulmonologists, emergency room physicians, and other health-care providers participating in the care of patients with
pneumothorax should become familiar with the ACCP consensus statement and the BTS guidelines in order to standardize the management approach. Although there are some limitations to these guidelines, they provide the basis on which to treat the majority of patients presenting with PSP and SSP (Figs. 58-5 and 58-6).
pneumothorax should become familiar with the ACCP consensus statement and the BTS guidelines in order to standardize the management approach. Although there are some limitations to these guidelines, they provide the basis on which to treat the majority of patients presenting with PSP and SSP (Figs. 58-5 and 58-6).
Table 58-4 Treatment Options for Pneumothorax | |
|---|---|
|
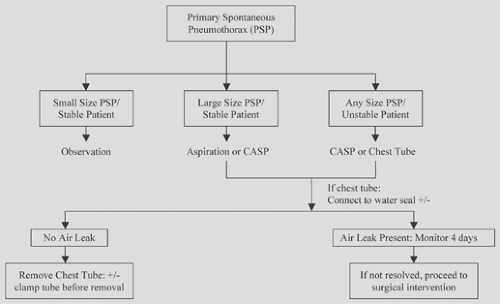 Figure 58-5. Management of first episode of primary spontaneous pneumothorax. CASP, catheter aspiration of pneumothorax (8.5–14 Fr). |
Oxygen and Observation
In 1993, the BTS was the first to issue management guidelines for PSP, strongly favoring a conservative approach, with emphasis on observation in select patients and simple aspiration as a technique that is simple, safe, and effective. The guidelines were updated in 2003.112 It is widely accepted that PSP presenting as a rim of <2 cm (approximately 30%) does not require intervention and should be considered for discharge, but this is an arbitrary figure, and the BTS document agrees with earlier clinical practice that observation is appropriate in greater degrees of collapse provided that the patient is only midly breathless (grade B) (Fig. 58-7). Some 70% to 80% of pneumothoraces estimated at <20% (1-cm rim) have no persistent air leak and recurrence after observation is less than in patients managed
with intercostal tube drainage.221 Observation of patients without significant breathlessness can occur in the hospital or in an outpatient setting after 6 hours of observation in the emergency department provided that the patient has easy access to medical care (good consensus, grade B).26,112 The great majority of patients with minor lung collapse have a closed pneumothorax, and it has been calculated that air reabsorbs at a rate of 2.2% per day with a tendency for larger pneumothoraces to reexpand at a faster rate.144 Absorption of air in the pleural space is accelerated three- to fourfold this rate by breathing an oxygen-rich mixture, thus increasing the gradient for nitrogen absorption, as demonstrated in rabbit and human studies.47,88,112,115,218 Oxygen supplementation in these studies varied from nasal cannula at 3 L/min to high-flow masks. Oxygen will also ameliorate the hypoxemia due to abnormal ventilation/perfusion relationships that may arise from a pneumothorax. The BTS guidelines note that patients with spontaneus pneumothorax admitted for care should receive high-flow (10 L/min) oxygen, with appropriate caution in COPD patients (grade B). The ACCP guidelines suggest observing the patient in a controlled setting for 3 to 6 hours and discharging him or her home if the repeat chest radiograph excludes progression (good consensus). This observation period helps ensure that the patient has a “closed pneumothorax,” one without active air leak—a point made by the BTS guidelines. Follow-up should occur within 2 days. Although the practice of outpatient observation for small PSPs has been criticized as being potentially dangerous based on a single case report, no fatalities have been recorded. Fatalities have been documented during inpatient observation, as a complication of treatment, or due to misdiagnosis or concomitant hemothorax.149,232 The ACCP and BTS guidelines reserve observation for clinically stable SSP patients with small
penumothoraces (<1 cm) and preferably in the hospital setting. Overnight observation is recommended also for patients who live remotely from the hospital and for those who refuse more invasive treatment (good consensus). Observation alone is inappropriate for symptomatic PSP or SSP (grade C).
with intercostal tube drainage.221 Observation of patients without significant breathlessness can occur in the hospital or in an outpatient setting after 6 hours of observation in the emergency department provided that the patient has easy access to medical care (good consensus, grade B).26,112 The great majority of patients with minor lung collapse have a closed pneumothorax, and it has been calculated that air reabsorbs at a rate of 2.2% per day with a tendency for larger pneumothoraces to reexpand at a faster rate.144 Absorption of air in the pleural space is accelerated three- to fourfold this rate by breathing an oxygen-rich mixture, thus increasing the gradient for nitrogen absorption, as demonstrated in rabbit and human studies.47,88,112,115,218 Oxygen supplementation in these studies varied from nasal cannula at 3 L/min to high-flow masks. Oxygen will also ameliorate the hypoxemia due to abnormal ventilation/perfusion relationships that may arise from a pneumothorax. The BTS guidelines note that patients with spontaneus pneumothorax admitted for care should receive high-flow (10 L/min) oxygen, with appropriate caution in COPD patients (grade B). The ACCP guidelines suggest observing the patient in a controlled setting for 3 to 6 hours and discharging him or her home if the repeat chest radiograph excludes progression (good consensus). This observation period helps ensure that the patient has a “closed pneumothorax,” one without active air leak—a point made by the BTS guidelines. Follow-up should occur within 2 days. Although the practice of outpatient observation for small PSPs has been criticized as being potentially dangerous based on a single case report, no fatalities have been recorded. Fatalities have been documented during inpatient observation, as a complication of treatment, or due to misdiagnosis or concomitant hemothorax.149,232 The ACCP and BTS guidelines reserve observation for clinically stable SSP patients with small
penumothoraces (<1 cm) and preferably in the hospital setting. Overnight observation is recommended also for patients who live remotely from the hospital and for those who refuse more invasive treatment (good consensus). Observation alone is inappropriate for symptomatic PSP or SSP (grade C).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree