Pneumonectomy
Robert J. Cerfolio
Ayesha S. Bryant
Pneumonectomy, defined as the removal of an entire lung, is technically one of the easiest operations and yet one of the riskiest operations performed in the chest. This risk is inherent to the final result of the procedure—having only one lung. Elective pneumonectomy when performed for nonsmall-cell lung cancer (NSCLC) has an operative mortality that ranges from 3% to 12%. This is significantly higher than that for an elective coronary artery bypass grafting. Unlike other paired organs that are removed for malignancy, the lungs are unusual in the fact that a right-sided pneumonectomy has a significantly higher operative risk than a left. The indication for pneumonectomy affects the operative risk as well. When a pneumonectomy is performed for a destroyed lung from an inflammatory process such as tuberculosis, the reported operative mortality is higher and ranges from 3% to 30%. The morbidity from this has been quoted as high as 44%. In this chapter, we focus on pneumonectomy for NSCLC and do not discuss the specific issues and considerations of pneumonectomy for mesothelioma, for destroyed lung, or other less common clinical scenarios. Finally, since many regard a pneumonectomy as a disease in and of itself, a surgeon rarely should go into an operation planning to do a pneumonectomy. Parenchymal preservation should always be front and center in the mind of the surgeon and a pneumonectomy should be done only if there exists no other way to completely remove all of the cancer and achieve negative margins. There are several different types of pneumonectomies. These various types are only mentioned for reasons of completeness but the description of most is out of the scope of this chapter.
TYPES OF PNEUMONECTOMIES
There are several different types of pneumonectomies with the most radical being extrapleural pneumonectomy, defined as the removal of the entire lung along with the ipsilateral pleura, hemidiaphragm, and hemipericardium. This type of operation is most commonly done on patients with mesothelioma. Other types of pneumonectomy include completion pneumonectomy, which is removal of the entire remaining lung after a patient has had some other portion of that lung removed at a previous operation. There are also intrapericardial and extrapericardial pneumonectomies. The former is often performed for anatomic reasons such as for large central tumors. A carinal pneumonectomy refers to the removal of an entire lung in addition to the carina. This requires an anastomosis between the remaining main stem bronchus and the distal trachea. This chapter focuses only on the surgical techniques of a standard pneumonectomy.
INDICATIONS FOR PNEUMONECTOMY
Prior to taking any patient to the operating room for a possible sleeve resection and/or what might turn out to be a pneumonectomy, the patient’s pulmonary function tests and cardiovascular status must be carefully assessed and a decision rendered regarding suitability. In addition, a pneumonectomy for a presumptive malignancy should never be performed until tissue confirmation has been obtained. Often the inability to perform a parenchymalsparing resection is discovered intraoperatively; so the preoperative evaluation should always include an assessment of the patient’s ability to tolerate a pneumonectomy. This possibility should be discussed with the patient preoperatively. The thoracic surgeon and the patient must weigh the risks and benefits of pneumonectomy, including increased morbidity and mortality and decreased exercise tolerance against the benefit of potential cure and increased survival.
We perform a cardiac stress test and an echocardiogram on all of these patients. Reversible cardiac ischemia is a contraindication to elective pneumonectomy; therefore, a thorough evaluation of the patient’s cardiac status must be undertaken. Patients should have areas of reversible myocardial ischemia revascularized and/or cardiologic clearance prior to elective pulmonary resection. On echocardiogram, the presence of a patent foramen ovale should be known preoperatively and significant valvular disease assessed and corrected if severe.
The baseline pulmonary function and the extent of the planned resection help guide the surgeon. Historically, there have been multiple articles proposing lower limits of various pulmonary function study variables below which operative risks become prohibitive. These limits should be used only as guidelines. However, a decision must be made for each patient on an individual basis. The old teaching that the FEV1 must be 800cc or greater at the completion of a pulmonary resection is obsolete. This old surgical dictum obviously does not take into account the size of a patient. An FEV1 of 800cc for a fourand-three-quarter-foot tall, thin female is more than adequate. Thus, the percent predicted FEV1 (FEV1%), which takes into account the various shapes and sizes that patients come in, is a much better representation. The percent postoperative predicted (popFEV1%) and percent postoperative predicted diffusion capacity of the lung for carbon monoxide (popDLCO%) have been shown to be reliable predictors of postoperative morbidity and mortality. These values should be calculated with the aid of a quantitative perfusion lung scan when patients have radiologic evidence of segmental, lobar, or greater areas of atelectasis. Important studies have shown that when the popFEV1% is less than 40, and the popDLCO% is less than 40, the operative risks are significantly increased. These values are guidelines since each patient and clinical situation is different.
Another important assessment besides the popFEV1% and the popDLCO% is the arterial blood gas measurement. The presence of significant hypercapnia raises an important red flag. When the preoperative arterial blood gas, a mandatory test prior to possible pneumonectomy, shows a PaCO2
of 48 mmHg or greater the operative risk is significantly increased. We have even lowered this in our practice to 45 mmHg. However, the surgeon and patient again must decide together before operation as to what will be done if a sleeve resection with parenchymal sparing is not technically feasible or if after the completion of a sleeve resection a positive bronchial margin is identified.
of 48 mmHg or greater the operative risk is significantly increased. We have even lowered this in our practice to 45 mmHg. However, the surgeon and patient again must decide together before operation as to what will be done if a sleeve resection with parenchymal sparing is not technically feasible or if after the completion of a sleeve resection a positive bronchial margin is identified.
Finally, since a pneumonectomy is associated with an increased risk compared to a lobectomy, certain oncologic principles need to be considered. The presence of residual or recalcitrant N2 disease (after neoadjuvant therapy for biopsy-proven N2 disease) is, in our opinion, an absolute contraindication for pneumonectomy (except in certain young patients with high popFEV1% and popDLCO% or those who are having significant hemoptysis, etc.). Once the decision has been made to perform a sleeve lobectomy or pneumonectomy, the operation should follow certain principles and be carried out in a certain sequence.
THE SURGICAL PROCEDURE: RIGHT-SIDED PNEUMONECTOMY
Despite the fact that there have been vast improvements in the field of general thoracic surgery and anesthesia, a pneumonectomy done today is not much different from the one done 30 years ago, except perhaps for the advent of stapling devices. After preoperative staging with the use of integrated positron emission tomography (PET)/computed tomography (CT) scan and CT scan, along with a careful assessment of the cardiopulmonary reserve, and the establishment of the absence of N2 disease via mediastinoscopy, transesophageal ultrasound with fine needle aspirate, or VATS, the patient is finally prepared for pneumonectomy. Preoperative bronchoscopy is performed and occasionally that alone can tell a surgeon that a pneumonectomy is required to achieve an R0 (negative margin) resection. If a lesion is attached (not invading, but attached) to the right proximal bronchial wall and one is able to get distal to it with the bronchoscope and it continues as one contiguous lesion down into the bronchus intermedius and into the right lower lobe, then right pneumonectomy is the only appropriate procedure. One must be sure that it is not simply tumor thrombus coming out of the right lower lobe bronchus, but rather is tumor adherent to the bronchial main stem wall. Similarly, if a lesion is quite large and involves the main pulmonary artery on the right and courses distally involving branches of the basilar pulmonary artery, a right pneumonectomy is required. Otherwise, in the vast majority of patients a sleeve resection should be the desired approach and the decision to do a pneumonectomy is not made on preoperative bronchoscopy, but rather on surgical exploration.
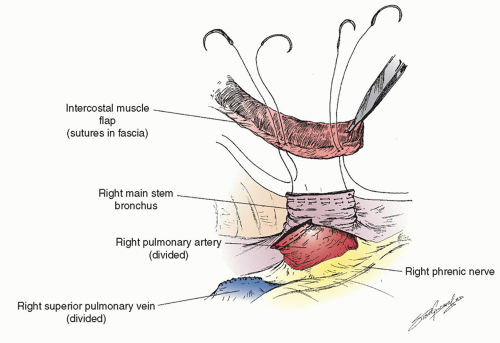
After the preoperative bronchoscopy the appropriate devices are placed— epidural catheter for postoperative analgesia (place prior to anesthesia), a double-lumen endotracheal tube (placed by the surgeon if there is a large and/or bloody lesion in the proximal airway to avoid any blood from spilling into the soon-to-beonly lung), an arterial line, central line, warming blanket, and serial compression devices on the legs. The patient is turned onto the left lateral decubitus position, carefully padded, and secured. There are several types of possible surgical approaches including a vertical axillary thoracotomy, and so on, but we focus on the most common approach for a pneumonectomy, a posterolateral thoracotomy. This can be performed in several ways as well, but we prefer cutting about half of the posterior aspect of the latissimus dorsi muscle, sparing all of the serratus anterior muscle and entering the chest over the uncut, unshingled, and unbroken sixth rib. We also strongly believe that an intercostal muscle flap should be harvested in any patient who is going to undergo a sleeve lobectomy or possible pneumonectomy (as shown in Fig. 6.1) for use either in covering a bronchial stump or wrapping around a bronchial anastomosis. We have demonstrated the usefulness of this pedicled graft in over 400 patients. It should be harvested and mobilized prior to the placement of a chest retractor. This provides a well-vascularized, soft, pliable muscle that is free of periosteum that does not calcify over time when harvested in this manner. It can reach to buttress any bronchus and it provides a barrier between sleeved pulmonary artery and/or bronchus though it should not be circumferentially wrapped around any anastomosis. It only takes a few minutes to mobilize and we have shown in a prospective randomized trial that it also is associated with a decrease in the pain of thoracotomy. If a sleeve resection is not performed, the muscle can then be tacked to the bronchus with the use of interrupted sutures as shown in Figure 6.2.
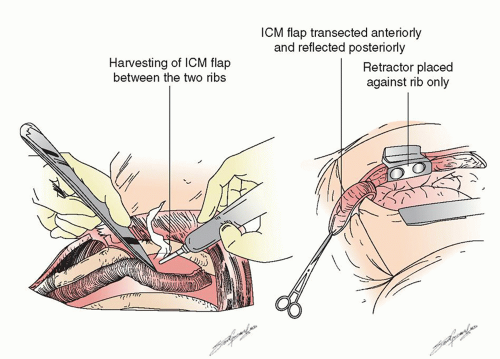 Fig. 6.1. Harvesting of an intercostal muscle off of the inferior aspect of the sixth rib and carefully off of the top bottom part of the fifth rib using a cautery so it is devoid of periosteum. |
Once the rib spreader is placed, the chest is explored and carefully inspected to rule out pleural effusion that could be consistent with T4 disease, metastatic nodules on the pleura or diaphragm that could represent M1 disease, or previously nonimaged pulmonary nodules. We prefer to remove all the mediastinal (N2) lymph nodes and perform a complete systematic thoracic lymphadenectomy as opposed to simple nodal sampling. If any of the N2 nodes look or feel suspicious or were imaged by CT and/or integrated
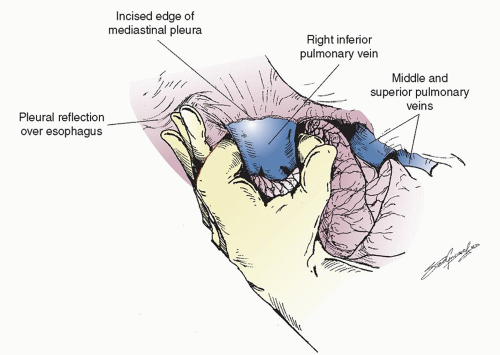
fluorine-18 fluorodeoxyglucose (FDG)-PET-CT as suspicious and were not ruled out as harboring cancer prior to thoracotomy, frozen section analysis should be performed. Once the decision has been made that resection is the appropriate option, the lung is retracted posteriorly and the anterior hilum is exposed. The hilar pleura is incised posterior to the phrenic nerve. The superior pulmonary and the inferior pulmonary vein are dissected free. Lymph nodes from this area are removed and the small veins that lead to the phrenic nerve are carefully coagulated so as not to injure the nerve since a functional phrenic nerve is important even following pneumonectomy. In rare situations, a single right pulmonary vein is found that makes a sleeve resection more difficult because the blood has to be baffled back into the left atrium. The inferior pulmonary ligament should have already been released during the inspection phase to allow removal of the level 9 and 8 lymph nodes. The inferior pulmonary vein is then easily encircled with an index finger as shown in Figure 6.3. If the subcarinal lymph nodes have been completely removed, this move is facilitated since the finger is placed in between the superior pulmonary vein and middle lobe vein and then comes around anterior to the right main stem bronchus. Dissection is then carried out anteriorly and more superiorly. Since a sleeve resection is usually attempted first, we often isolate the superior pulmonary vein next, depending on the size and location of the tumor and remove the N1, level 10, and level 11 lymph nodes. Very commonly, there is a large hilar lymph node that interdigitates between the superior aspect of the superior pulmonary vein and the main pulmonary artery just as it courses distally to the superior vena cava. If one is able to completely remove this large N1 node, the course of the distal main pulmonary artery becomes visible. This makes encircling the superior pulmonary vein safer and easier. The vein is carefully encircled around its posterior aspect so as not to injure the main pulmonary artery that runs just beneath it. If the tumor is large and proximal, the pulmonary artery may have to be encircled as the initial maneuver. At times it may be necessary to obtain control of the intrapericardial portion of the right main pulmonary artery. The pericardium should be opened away from the phrenic nerve,
which must be visualized and spared. Opening the pericardium allows central inspection to assess resectability and allows for proximal control of the vessels. However, the intrapericardial mobilization may increase the incidence of postoperative atrial fibrillation (AF). A finger is then used to encircle the main pulmonary artery and the superior pulmonary vein.
fluorine-18 fluorodeoxyglucose (FDG)-PET-CT as suspicious and were not ruled out as harboring cancer prior to thoracotomy, frozen section analysis should be performed. Once the decision has been made that resection is the appropriate option, the lung is retracted posteriorly and the anterior hilum is exposed. The hilar pleura is incised posterior to the phrenic nerve. The superior pulmonary and the inferior pulmonary vein are dissected free. Lymph nodes from this area are removed and the small veins that lead to the phrenic nerve are carefully coagulated so as not to injure the nerve since a functional phrenic nerve is important even following pneumonectomy. In rare situations, a single right pulmonary vein is found that makes a sleeve resection more difficult because the blood has to be baffled back into the left atrium. The inferior pulmonary ligament should have already been released during the inspection phase to allow removal of the level 9 and 8 lymph nodes. The inferior pulmonary vein is then easily encircled with an index finger as shown in Figure 6.3. If the subcarinal lymph nodes have been completely removed, this move is facilitated since the finger is placed in between the superior pulmonary vein and middle lobe vein and then comes around anterior to the right main stem bronchus. Dissection is then carried out anteriorly and more superiorly. Since a sleeve resection is usually attempted first, we often isolate the superior pulmonary vein next, depending on the size and location of the tumor and remove the N1, level 10, and level 11 lymph nodes. Very commonly, there is a large hilar lymph node that interdigitates between the superior aspect of the superior pulmonary vein and the main pulmonary artery just as it courses distally to the superior vena cava. If one is able to completely remove this large N1 node, the course of the distal main pulmonary artery becomes visible. This makes encircling the superior pulmonary vein safer and easier. The vein is carefully encircled around its posterior aspect so as not to injure the main pulmonary artery that runs just beneath it. If the tumor is large and proximal, the pulmonary artery may have to be encircled as the initial maneuver. At times it may be necessary to obtain control of the intrapericardial portion of the right main pulmonary artery. The pericardium should be opened away from the phrenic nerve,
which must be visualized and spared. Opening the pericardium allows central inspection to assess resectability and allows for proximal control of the vessels. However, the intrapericardial mobilization may increase the incidence of postoperative atrial fibrillation (AF). A finger is then used to encircle the main pulmonary artery and the superior pulmonary vein.
The sequence of ligation of the hilar structures is highly dependent on the position of the lesion and the surgeon’s preferences. Prior to taking any vessels, we have shown in a nonrandomized but prospective trial that giving 250 mg of Solu-Medrol may help prevent the complication of postpneumonectomy pulmonary edema (PPE). Thus, we recommend giving this 5 to 10 minutes prior to ligating the vessels. One should also test clamp the pulmonary artery for a minute or two and ensure that the patient’s hemodynamics tolerate the shunting of all of the pulmonary blood supply into the left lung. If the patient’s blood pressure drops quickly and if the clamp or the fingers/hand used to clamp the pulmonary artery is not compressing the heart, this suggests that the patient will not tolerate a pneumonectomy. This move should be repeated several times, but if it continues to occur the vessels should not be taken and the operation should be aborted. This is a very rare occurrence but is a final check of the patient’s suitability for pneumonectomy. In this situation, despite the careful preoperative evaluation that suggested that the patient was a candidate for pneumonectomy, the procedure cannot be carried out.
There is little data that documents the advantage or disadvantage of taking the vein prior to the artery. Kurusu has reported an oncologic advantage in taking the vein prior to the artery because it may prevent the accidental dislodgement of cancer cells into the systemic circulation during the manipulation and dissection of the tumor. Others believe there is less blood lost in the removed lung when the vein is taken after the artery. There is no data to support these latter claims. However, if a pneumonectomy (or lobectomy) is performed robotically, there is distinct advantage to taking the vein last. This prevents engorgement of the specimen. If the lung to be removed becomes swollen from early vein ligation, it leads to more bleeding from the specimen, less working room in the chest during minimally invasive surgery, and more difficulty in manipulating the specimen from one side to the other.
If a thoracotomy is the chosen surgical approach then the vessels can also be ligated in several different ways. We prefer to take the vein with a vascular stapler that staples and cuts simultaneously, but prefers to take the artery with a vascular stapler that only staples. The staplers disperse the closure of the vessel over its entire length as opposed to bunching it up into one suture ligature. Another method involves clamping the artery, cutting, and then oversewing the end with a running Prolene suture (Ethicon, Cincinnati, Ohio) along its length. This obviously takes longer to do.
Once the vessels have been cut, the bronchus is the only remaining structure that is keeping the lung in vivo. We prefer not to take the bronchus first on a right or left pneumonectomy as we often do on a right upper lobectomy because it is easier to achieve a short stump if the bronchus is taken after the vessels are divided. However, on the right side the bronchus can be taken prior to the vessels if needed to obtain better access to the pulmonary artery. It can make the dissection easier for large anterior tumors that are too big to allow for anterior dissection of the hilar structures. It can be taken with a knife or a stapler (we use 4.8-cm-long staples) before the artery or veins or divided. If taken with a knife, one can visualize the trachea and ensure the position of the double-lumen tube. If taken with a stapler, one should ask the anesthesiologist to inflate and then deflate the balloon on the tracheal cuff side after closing the stapler but before firing the instrument to ensure that the tracheal balloon of the double-lumen tube has not been entrapped. Either way a short stump is a crucial and mandatory part of a successful right pneumonectomy.
We divide the azygous vein first with a vascular stapler prior to stapling the right main stem bronchus. One must ensure the central venous line is not in the azygous vein. It is usually very easy to see the blue tip of the central line in the superior vena cava or even in the azygous vein. In rare situations when an intercostal muscle is not available and if there is no pericardial fat pad, the azygous vein can be used as a form of bronchial coverage. This is accomplished by dividing the vein as far posteriorly as possible and then ligating the other end tied flush against the superior vena cava. The vein is cut as distally as possible and this provides a long flap of a defunctionalized vein that can be split and used to cover the right main stem bronchus. We frequently use this technique for a radical extrapleural pneumonectomy for mesothelioma because the intercostal muscle is not useable as it may be infiltrated with tumor cells. It is important to note that a pleural flap does not provide adequate coverage since it is paper thin and is often nonviable within 48 hours after surgery.
Once the pulmonary vessels have been ligated and divided, the bronchus is taken last. The lung is retracted so the right main stem is exposed. If all of the subcarinal lymph nodes have been dissected and the large bronchial artery that comes from the undersurface of the carina to the subcarinal nodes has been clipped, the left main stem bronchus is already exposed. A stapler is placed (more easily on the right than on the left because of the absence of the aortic arch on the right in most patients) and fired flush with the trachea. As described above, one must ensure that there are no parts of the double-lumen tube in the staple line and that no suction catheters are within the bronchus before stapling and cutting. Although there is some literature about handsewing the bronchus, there is no real data that suggests that handsewing is better than stapling or vice versa. We recommend the stapling technique.
The bronchial margin should be sent for frozen section analysis as should the artery or vein margin if the tumor is close. The chest is then filled with warm water, not saline. We prefer the former because of the oncologic possibility that it may lyse tumor cells that are floating in the chest. Also it is easier to see through it than saline to check the stump for a leak. The double-lumen tube should now be opened to both chests and the stump is tested. If a bronchial leak is noted, nonabsorbable monofilament sutures are placed under the staple line in a vertical mattress fashion with knots opposite the pulmonary artery. The bronchial closure should be interrogated again to assure that there is no stump leak. The intercostal muscle flap is then set in place to cover the entire bronchus (as shown in Fig. 6.2). The sutures that are placed in the bronchus should be small superficial bites so as not to injure the stump or its blood supply. The entire stump is covered. We have used this method to cover the bronchus after lobectomy and main stem bronchus after pneumonectomy in over 500 patients, including over 100 who have had preoperative radiation and have had only two BPFs.
We prefer leaving a chest tube in the postpneumonectomy space mainly to monitor postoperative bleeding but also to allow for setting the mediastinum. The best way to handle the postpneumonectomized space has never been described. Some surgeons recommend aspirating the air out with a syringe after the chest has been closed, just prior to leaving




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree