Pneumonectomy and Its Modifications
Stanley C. Fell
Malcolm M. DeCamp Jr.
Evarts Graham performed the first successful one-stage left pneumonectomy in April 1933 on James Gilmore, a 48-year-old physician with a biopsy-proven carcinoma of the left upper lobe.5,8 Graham had planned a lobectomy, but the operative findings were that the carcinoma extended into the left lower bronchus. Graham decided that pneumonectomy was the only acceptable procedure.
He initially occluded the pulmonary hilum with a catheter to assess the effects of sudden obstruction of the pulmonary artery, fearing that the symptoms of pulmonary embolism might ensue. The patient remained stable, however, and Graham proceeded with pneumonectomy by mass ligature of the hilum using chromic catgut. He also inserted seven radon seeds of 1.5 mCi each into the hilar stump. Horrified by the size of the empty pleural space, he performed a seven-rib thoracoplasty and closed the chest with catheter drainage. Gilmore’s pathology specimen was subsequently reviewed and noted to be T2, N1, stage IIB.
The following month, Graham presented the case at the sixteenth Annual Meeting of the American Association for Thoracic Surgery in Washington, D.C. The case report was published by Graham and Singer8 in 1933 and also subsequently by Graham9 in 1949. Gilmore actually resumed practice and lived for 30 more years, ultimately dying of cardiac and renal disease. Ironically, he outlived Graham, who succumbed to widely metastatic lung cancer in 1957.
Graham’s presentation stimulated Archibald in Montreal, who performed a left pneumonectomy by hilar dissection in July 1933. Archibald closed the bronchus with a twisted silver wire. In November 1933, Overholt,3 in Boston, performed the first successful right pneumonectomy by hilar dissection. Two weeks after Archibald’s successful case, Rienhoff14 at Johns Hopkins performed the first pneumonectomy by hilar dissection and closure of the bronchus with interrupted sutures, a technique employed to this day.
Indications for Pneumonectomy
Currently, pneumonectomy is performed for carcinoma of the lung, usually bulky and centrally located. Occasionally, pneumonectomy is indicated for a destroyed lung secondary to chronic infections such as tuberculosis, atypical mycobacterial infection, bronchiectasis, and fungal disease.
All patients require a thorough evaluation to rule out metastatic disease and ascertain the patient’s physiologic capacity to withstand the resection (see Chapters 20 and 21). Pneumonectomy should never be performed in the absence of a definite histologic diagnosis.
Overview
The key tenet for a safe pneumonectomy is proper exposure. The customary approach is a posterolateral thoracotomy that enters the chest through the fifth interspace or the bed of the subperiosteally resected fifth rib. The operation can also be carried out using a muscle-sparing incision provided that exposure is adequate (see Chapter 25). Video-assisted pneumonectomy has been reported but should be employed only by surgeons with significant experience with this technique (see Chapters 33 and 35). Single-lung ventilation using either a double-lumen tube or a bronchial blocker has made pneumonectomy a technically easier procedure and should be used if possible.
Upon entering the chest, resectability is first assessed, as described by Fry.7 Inspection of the pleural space to rule out intrapleural metastases should be done and suspicious areas resected for biopsy. Locoregional spread, including invasion of major vascular and mediastinal structures such as the aorta, ventricles, esophagus, and spine, precludes complete resection in virtually all instances and negates any benefit from proceeding with pneumonectomy. Mediastinal lymph nodes should be fully staged whether or not mediastinoscopy has been performed. This includes biopsy of paratracheal (levels 2 and 4), aortopulmonary window (level 5), prevascular (level 6), subcarinal (level 7), and pulmonary ligament (level 9) lymph nodes, as described by Naruke and associates13 and revised by Mountain and Dresler.12 Any other suspect nodes should also be sampled. Controversy persists over formal mediastinal node dissection versus sampling. The American College of Surgeons Oncology Group has completed a trial comparing the two procedures. Results await mature follow-up to assess for survival differences
between the two techniques. In patients who have undergone induction therapy for clinical N1 or N2 disease, mediastinal lymphadenectomy is warranted. Any approach to the lymph nodes short of systematic sampling is unacceptable. Multistation involvement and extracapsular spread indicate that complete and therefore curative resection is not possible—again, a contraindication to pneumonectomy.
between the two techniques. In patients who have undergone induction therapy for clinical N1 or N2 disease, mediastinal lymphadenectomy is warranted. Any approach to the lymph nodes short of systematic sampling is unacceptable. Multistation involvement and extracapsular spread indicate that complete and therefore curative resection is not possible—again, a contraindication to pneumonectomy.
Finally, the surgeon must carefully evaluate the need for pneumonectomy. Occasionally intraoperative findings allow lesser resections—such as sleeve resection of the bronchus and/or pulmonary artery—to be performed, allowing either the upper or lower lobe to be spared, as reported by Jensik and asso- ciates10 and subsequently confirmed by others (see Chapter 29).
The usual sequence of management of the hilar structures is artery, vein, and bronchus. However, circumstances may dictate that a different sequence be employed. For example, initial division of the bronchus facilitates exposure of the pulmonary artery. In instances where identification of the hilar structures is obscured because of inflammation, transection of the inferior pulmonary vein first will often facilitate identification and dissection of the remaining hilar structures. Tumor encroachment or invasion of a pulmonary vein should lead the surgeon to divide the veins first, often intrapericardially, to prevent tumor embolization. Some surgeons prefer to control the veins first, based on the theory that this might lessen hematogenous spread of malignant cells. Contrary to older beliefs, the lung does not become engorged if the venous outflow is occluded before the artery.
Technique
Left Pneumonectomy
Hilar dissection is best performed using scissors. The mediastinal pleura is circumferentially incised around the hilum, and the lymph nodes are evaluated as previously described. The phrenic nerve is preserved and not sacrificed unless it is directly invaded by tumor in an otherwise resectable situation. Dissection of the mediastinal fat and lymph node–bearing tissue around the main pulmonary artery is performed. The artery is then freed in the subadventitial plane (Fig. 30-1). Division of the vagal branches to the posterior wall of the bronchus allows exposure of this structure. The areolar tissue between the anteromedial wall of the bronchus and pulmonary artery is now divided (Fig. 30-2). Pledget and finger dissection using the thumb and forefinger is used to encircle the artery (Fig. 30-3). A vessel loop is placed around the artery, and it can be occluded with a vascular clamp, tourniquet, or manually to assess the patient’s hemodynamic stability. Hypotension, tachycardia, and right ventricular dysfunction or dilation are indications that the patient will not physiologically tolerate a pneumonectomy.
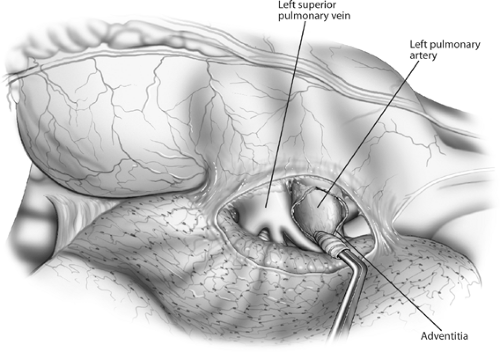 Figure 30-1. Anterior aspect of the left hilum. The mediastinal pleura has been incised and the pulmonary artery rolled out of its adventitia, separating it from the superior pulmonary vein. |
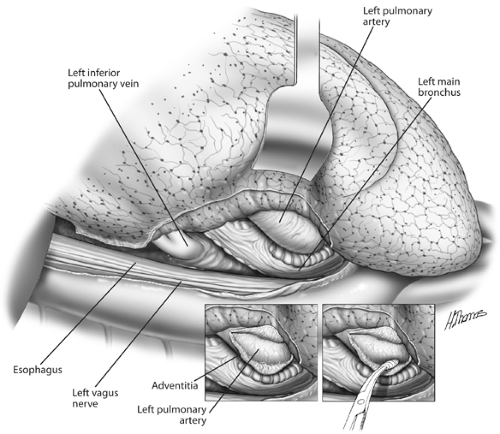 Figure 30-2. Posterior aspect of the left hilum. The pulmonary artery is being separated from the left bronchus. |
If further length of the artery is required, dissection may be carried proximally, transecting the ligamentum arteriosum and avoiding injury to the left recurrent laryngeal nerve. Additional length can be obtained by carrying out intrapericardial dissection of the artery, as described by Allison1 in 1946. The pericardium anterior to the phrenic nerve and just superior to the entrance of the superior pulmonary vein is opened and extended superiorly to the inferoanterior edge of the pulmonary artery. By sharp dissection, the artery can be freed from its pericardial investments. If the artery is to be divided at its origin, care should be taken not to compromise the lumen of the main pulmonary artery, an event that would lead to obstruction of the right ventricular outflow tract.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


