Chapter 69 Plastic Surgery
Reconstructive Techniques
Skin Grafts
The take of either type of skin graft occurs in three phases:
Skin Flap Surgery
Skin Flaps
Muscle and Musculocutaneous Flaps
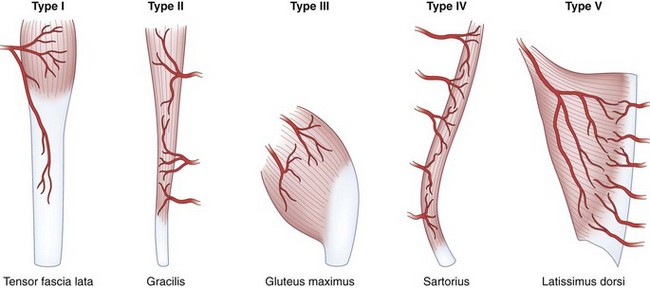
Muscle flaps are classified according to their principal means of blood supply and the patterns of vascular anatomy (Fig. 69-1):
Fascia and Fasciocutaneous Flaps
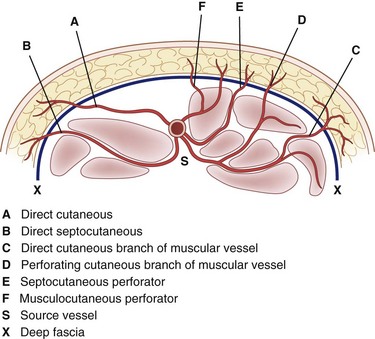
The anatomic features of a fasciocutaneous flap are the fascial feeder vessels, also called the fascial perforators, which are branches of source vessels to a given angiosome. An angiosome is the three-dimensional block of tissue supplied by a source artery; the entire surface of the body is comprised of a multitude of angiosome units. The fascial feeder vessels do not perforate the deep fascia, but terminate within the fascial plexus. The fascial plexus is not a structure but is a confluence of multiple adjacent vascular intercommunications that exist at the subfascial, fascial, suprafascial, subcutaneous, and subdermal levels (Fig. 69-2).
The design of fasciocutaneous flaps has been learned by experience and the limits of these flaps still remain to be discovered. There are no set rules, because deep fascial perforators are frequently anomalous in caliber and location, not only among individuals but also on opposite sides of the same person. The expected range of flap size is learned through the experience of other surgeons.1,2
Perforator Flaps
The use of perforator flaps continues to evolve. Current work includes flap thinning, a technique for removing excess adipose tissue from the perforator flap as it is raised. This would provide a large delicate segment of vascularized skin for reconstruction in areas such as the ear, in which contour is important. Another innovation is the discovery of new flaps based on perforators smaller than 0.8 mm in diameter found superficial to the fascial plane. By eliminating the dissection needed to trace a perforator through the muscle, operating time is shortened and there is potential for developing a much larger number of suitable flaps. The challenge with these suprafascial free flaps is the supermicrosurgery needed for anastomoses in such small vessels.3
Microvascular Free Tissue Transfer
Tissue survival rates for free tissue transfers exceed 95%. Reexploration rates range from 6% to 25% and thrombosis of the arterial anastomosis is the most common finding at reoperation. This is termed primary thrombosis when technical faults lead to anastomotic failure. These faults include narrowing of the lumen, sutures tied too loosely so that media of the vessel is exposed in the gap and clot forms, sutures tied too tightly that tear through the vessel, too many sutures with subendothelial exposure and clot formation, and sutures that inadvertently take a bite of the back wall of the vessel, which obstructs the lumen. The term secondary thrombosis refers to kinking or compression of vessels by hematoma or edema, which leads to decreased inflow. With reexploration, salvage rates have been seen to vary from 54% to 100% in different series.4
Supermicrosurgery
The introduction of supermicrosurgery, which allows the anastomosis of smaller caliber vessels and microvascular dissection of vessels ranging from 0.3 to 0.8 mm in diameter, has led to the development of new reconstructive techniques. Free perforator-to-perforator flaps using suprafascial vessels can be transferred more quickly and the tissue can be obtained from better concealed parts of the body.5 If a discrete perforator can be identified anywhere on the body, a flap can be designed around it. This has been called a “freestyle flap.” The constraints of using only described territories can be disregarded and the donor site selected solely on the basis of the best possible match for color, contour, and texture at the recipient site. Disadvantages are the anatomic variation of the perforators and the need for supermicrosurgical technique. The latter includes the use of 12-0 nylon sutures with 50- to 30-µm needles; the surgeons who pioneered “freestyle reconstruction” have noted that it is difficult to learn and can be tedious.6
Tissue Expansion
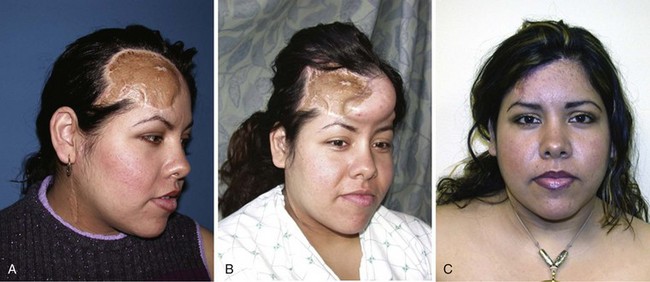
Expanders should be placed under tissue that best matches the lost tissue (Fig. 69-3). Normal landmarks such as the eyebrow or hairline should not be distorted. The incision to insert the expander can be placed at the edge of the defect that later will be excised, because a scar in this position will be removed at the time of the next surgery. The most common reason for expander failure is construction of a pocket that is too small for the device. An expander with a curled edge may later protrude through the incision or erode through the overlying tissue. Filling of the expander is initiated approximately 2 weeks after surgery and continued at weekly or biweekly intervals. The rate of expansion is limited by the relaxation and growth of the tissue overlying the expander. Pain and palpable tightness over the expander are clinical indicators that guide the rate of expansion. The patient is ready for the second surgical procedure when the expanded tissue is adequate to produce the desired effect. If the flap is to be advanced, it must be measured to ensure that it is large enough and has the correct geometry to cover the defect. At the second surgery, the skin is incised through the old scar, the capsule around the expander is opened, the expander is removed, and the expanded flap is advanced over the defect. It is important to confirm that the expanded tissue will replace the defect before excising the defect. If it is not sufficient, this is handled by subtotal resection of the defect and leaving the expander in place for a second round of expansion.7
Pediatric Plastic Surgery
Craniofacial Surgery
Craniofacial Microsomia
Craniofacial microsomia, also known as hemifacial microsomia, is a constellation of abnormalities involving deficient development of parts of the face related to the first and second branchial arches.8 Deformity can be unilateral or bilateral and can involve the orbit, mandible, external ear, facial nerve, and facial soft tissue. Each or all of the structures may be involved and to varying degrees. The cause is unknown but is thought to be related to in utero vascular compromise of the stapedial artery. Treatment of craniofacial microsomia is complex and the approach has to be tailored for individual patients. Functional problems such as airway compromise or eye exposure are treated in childhood; reconstruction of other structural defects is delayed until the patient is almost full-grown.
Cleft Lip and Palate
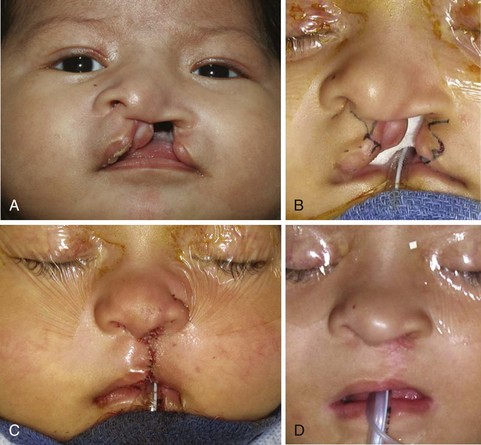
There are many techniques for repair of a cleft lip, but most are a variation of the rotation advancement repair. Millard introduced this technique of downward rotation of the medial portion of the lip and advancement of the lateral portion into the defect created by the rotation. The repair is based on the principle that existing elements need to be returned to their normal position to restore the normal anatomy while remaining cognizant of future growth and the effects of surgery on growth (Fig. 69-4).9
Other procedures are indicated for some patients, but this generally cannot be predicted in infancy. Approximately 15% of patients will continue to demonstrate velopharyngeal insufficiency after initial palate repair and secondary palatal lengthening or other approaches to promote velopharyngeal closure are indicated, typically after 3 years of age.10 Septorhinoplasty is usually necessary to correct residual nasal deformity in the teenage years after final dental restoration and orthodontics. A subset of unilateral cleft lip and palate patients will develop maxillary hypoplasia that is iatrogenic and related to scarring and growth retardation from lip and palate surgery. Depending on the degree of maxillary hypoplasia, LeFort I maxillary advancement in the teenage years may be indicated. In sum, treatment of a child born with a cleft lip and palate does not end after palate repair, but rather requires observation by a craniofacial team throughout development into adulthood and must be tailored for each individual.
Melanocytic Nevi
Congenital melanocytic nevi are hamartomas consisting of nevus cells. Nevi are classified by size—small (<1.5 cm), medium (1.5 to 19.9 cm), large (>20 cm), and giant (>50 cm). The classification dictates the prognosis and reconstructive approach. Risk of melanoma occurring in a melanocytic nevus varies by report, but is estimated to be less than 5% in small or medium lesions and typically presents after puberty. In large and giant nevi, the reported risk of melanoma development is up to 10%.11 Unlike the case for small or medium nevi, malignancy in large and giant nevi typically occurs in the first 3 years of life. Large and giant nevi also have an increased incidence of leptomeningeal involvement that can be diagnosed by magnetic resonance imaging (MRI). In addition, psychosocial and developmental issues associated with larger nevi are significant, so early excision and reconstruction are recommended for large and giant nevi.
Plastic Surgery of the Head and Neck
Maxillofacial Trauma
Emergent Management
Life-threatening hemorrhage, defined as 3 U of blood loss or hematocrit below 29%, occurs in a small percentage of facial trauma patients. In most cases, bleeding is effectively controlled with pressure, packing and, in the case of significant soft tissue avulsion, the rapid placement of temporary bolster sutures. Blind attempts to clamp and ligate vessels should be avoided because this is usually unnecessary and may result in injury to critical structures, such as the facial nerve. With penetrating trauma, hemorrhage is controlled in the operating room with vessel identification and ligation and, if unsuccessful, by angiographic selective embolization. With blunt trauma, severe hemorrhage is usually from the internal maxillary artery. The most effective way to control bleeding, especially when it is associated with midfacial fractures, is fracture reduction and stabilization. This can be accomplished quickly by temporary placement in maxillomandibular fixation using rapid techniques such as fixation screws. Severe hemorrhage from skull base and nasoethmoid fractures can often be controlled with anteroposterior nasal packing. Placement of Foley balloon catheters in each nasal airway serves to tamponade the bleeding and also stabilizes the packing. Current protocols for control of hemorrhage in blunt facial trauma settings involve selective angiography if these measures fail (Fig. 69-5).12 Angiographic embolization is effective but is associated with significant morbidity, including the possibility of stroke or necrosis of midfacial structures such as the palate. In unstable patients, fracture reduction and nasal packing may be attempted on the angiography table to be followed immediately with embolization, if necessary.
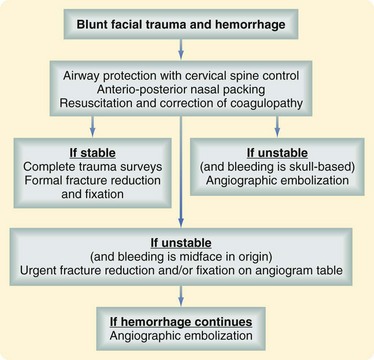
FIGURE 69-5 Algorithm for the management of life-threatening hemorrhage in the setting of blunt facial trauma.
(Adapted from Ho K, Hutter JJ, Eskridge J, et al: The management of life-threatening haemorrhage following blunt facial trauma. J Plast Reconstr Aesthet Surg 59:1257–1262, 2006.)
Evaluation and Diagnosis
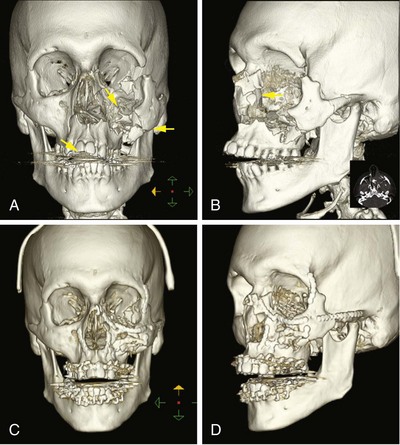
The primary diagnostic studies for facial injury are physical examination and CT. Systematic physical examination can detect deformity, soft tissue injury, cerebrospinal fluid (CSF) leak, and facial nerve injury. Palpation is used to identify bony stepoffs or midface instability. The eyes are examined for proptosis or enophthalmos, extraocular muscle function, and visual acuity. In patients who cannot cooperate with a physical examination and for whom there is reasonable suspicion of periorbital injury, a forced duction test should be performed. The occlusion is evaluated for subjective or objective malocclusion. Extraocular muscle entrapment, acute enophthalmos, and malocclusion are indications that surgical treatment of facial fractures will be required. Fine-cut CT of the face with direct or reformatted coronal and sagittal views is used to diagnose facial trauma and direct nonsurgical and surgical treatment (Fig. 69-6). With current CT scanning technology, plain films are not necessary and provide less information. An exception is the Panorex, which is used by many physicians as an adjunct or primary study for mandible fractures and to assess teeth and their roots in particular.
Craniofacial Fractures
Maxillary fractures are classified using the LeFort system based on the level at which the midface is separated from the rest of the craniofacial skeleton. Repairs focus on the restoration of facial height and projection. With significant comminution or bone loss, bone grafting may be required to maintain the appropriate position of the maxilla in space. Rigid plate and screw fixation obviates the need for prolonged maxillomandibular fixation. Fractures of the mandible are treated by reduction and rigid fixation using restoration of occlusion as the principle intraoperative and postoperative goal. Many mandibular fractures are treated with open reduction and internal fixation, which may make maxillomandibular fixation unnecessary.13 Certain fractures, however, are best treated closed, and the decision to pursue an open or closed approach depends on the fracture location and orientation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree