Plain Film Examination of the Chest
Lawrence M. Boxt
Overview
The value of plain film examination of the heart and lungs lies in its availability, long history of clinical utility, and ability to display acute and chronic changes in a safe and convenient manner. Diagnosis is based on identification and characterization of variance in the appearance of structures on chest film from their expected normal appearance. Interpretation of the chest radiograph involves systematic evaluation of the quality of the examination, the position of the patient undergoing examination, as well as the radiographic changes encountered in the examination. The validity and significance of observed abnormalities depend on a belief in the physiologic or technical basis for their presence. Attention to these factors allows extraction of pertinent, reliable evidence of pathophysiologic mechanisms that allows the observer to make cardiovascular diagnoses, as well as to assess the instant physiologic status of the patient at the time of examination.
Glossary
Cardiac contour
The borders of the cardiac silhouette are formed by portions of the cardiac chambers and aorta and pulmonary artery; in oblique view, different border-forming chambers are accentuated or brought into view. Analysis of chamber and great artery abnormality is based on systematic estimation of the appearance of these contours. Evaluation begins by dividing the cardiac silhouette into the left and right heart borders. The left heart border is composed of the aortic arch, main pulmonary artery, left atrial appendage, and left ventricular portion. The right heart border is composed of the superior vena cava, ascending aorta, and right atrial portion.
Cardiac position
The position of the heart within the chest. In general, it is determined by estimating where the bulk of the cardiac silhouette resides. We expect the cardiac apex and bulk of the ventricular myocardium to lie just slightly off the midline, toward the left (levocardia). Thus, mesocardia and dextrocardia describe the heart as straddling over toward the right with respect to an imaginary line drawn in the chest midline. Dextrocardia refers to the presence of a heart in the right chest resulting from an abnormality of embryologic cardiac rotation. It is frequently associated with congenital heart disease. Dextroposition describes a heart in the right chest, caused by noncardiac abnormalities that affect the normally formed heart.
Cardiac rotation
The heart and proximal great arteries are contained by the pericardium and fixed in the superior mediastinum and at the diaphragm. Right atrial, ventricular, or both atrial and ventricular dilatation produces (looking from below) clockwise rotation of the heart and great vessels, so that the appearance of the left heart border in posteroanterior (PA) view is altered. The superior mediastinal silhouette is narrow as the superior vena cava rotates toward the midline, and the midleft heart border becomes more convex as the dilated right ventricular outflow forms the left heart border.
Left ventricular configuration
Hearts that appear unusual may be morphologically normal. However, many abnormal hearts share a common appearance: The contour of the left ventricular portion of the left heart border appears greater in curvature than expected. This appearance of the left-sided heart contour is found in a variety of congenital and acquired forms of heart disease. It is a sensitive “case finder” or indicator of cardiac abnormality. However, it is nonspecific and requires appreciation of other radiographic or clinical findings for more precise cardiac diagnosis.
Pulmonary edema
Characteristic, inhomogeneous-appearing infiltrates found with alveolar edema representing the severe end of the spectrum of the radiographic changes of pulmonary venous hypertension. The earliest radiographic finding in elevated left atrial pressure is indistinct lower lobe pulmonary vessels.
Pulmonary vascularity
The radiographic appearance of the pulmonary arteries and veins closely correlates with left atrial pressure and the regional distribution of water in the lungs. This general term refers to the caliber of the larger pulmonary arteries and veins in a chest radiograph. Comparison
of left-sided vessels with those on the right, upper with lower vessels, and central with peripheral vessels is commonly used to differentiate failure from pneumonia and shunt from pulmonary hypertension.
of left-sided vessels with those on the right, upper with lower vessels, and central with peripheral vessels is commonly used to differentiate failure from pneumonia and shunt from pulmonary hypertension.
Shunt vascularity
This subcategory of pulmonary vascularity refers to the status of the pulmonary arteries in individuals with left-to-right shunts. In particular, it refers to increase in the caliber of the entire pulmonary arterial tree, from main pulmonary artery segment, to peripheral subsegmental pulmonary arterial branches. Vessels are sharp, demonstrate normal peripheral branching, and extend further toward the pleura.
Introduction
Plain film examination of the chest is nearly always employed to determine the presence of heart disease and to help establish its etiology and severity. The development and use of angiographic, nuclear, and tomographic cardiac imaging technologies (echocardiography, computed tomography, and magnetic resonance imaging) have changed but not excluded the role of plain film radiography in the management of these patients. Characteristic changes in the atrial and ventricular portions of the cardiac contour reflect change in their chamber volume and myocardial mass. Similarly, changes in the contours formed by the great arteries and veins of the chest reflect altered pressure and blood flow. Evaluation of these changes provides the substrate for constructing a differential diagnosis or to assess the severity and often etiology of the cardiac dysfunction.
This chapter is designed to assist clinicians in the use of plain film examination of adult patients with acquired and congenital heart disease. Conventionally, chapters describe radiographic abnormalities in an organized list of cardiovascular conditions. This format can be excellent if the reader knows the answer before asking the question (i.e., if you know the patient has mitral stenosis, then reference to such a chapter provides a source for review of the literature concerning radiographic diagnosis and so on). However, as is more often the case, one does not know the plain film diagnosis; rather, one has acquired a list of positive (and negative) radiographic findings and is in need of an outline of differential diagnoses. In other words, the role of the chest film examination has changed in the management of patients with cardiovascular disease. The examination is performed more to evaluate the instant physiologic status of the patient. This change in use is not surprising, considering the rapid advances in noninvasive cardiovascular imaging. On the other hand, there remains a great deal of diagnostic information contained in plain film examination that can and does benefit the clinician.
This chapter is organized based on radiographic abnormality (i.e., the cardiac silhouette in the PA chest film is divided into left and right heart borders and the pulmonary vascularity). The left heart border contains the aortic arch, pulmonary artery, left atrial appendage, and left ventricular contours. The right heart border contains the superior vena cava, ascending aorta, and right atrial contours. The parenchymal pulmonary vascularity is described in terms of the appearance of the pulmonary vessels. Thus, entrée into cardiovascular diagnosis and differential diagnosis is based on identification of an abnormality or constellation of abnormalities. This approach is useful, especially if one does not know the diagnosis before interpretation of the examination.
After describing the normal appearance of the cardiopulmonary structures, the range of abnormalities of that portion of the chest film is characterized and the differential diagnosis of congenital and acquired cardiovascular disease based on those specific changes is presented. That is, many different diseases may produce individual specific changes in the chest film, but more specific diagnosis may be made or a differential diagnosis ordered by evaluation of the particular constellation of findings present. In other words, rather than review the radiographic findings of a series of cardiovascular lesions, particular radiographic abnormalities are described, how these changes may represent the effect of a congenital abnormality or acquired pathophysiologic mechanism is explained, and then these findings are used to construct a differential diagnosis. This is a clinically relevant approach, and it more effectively demonstrates the utility and limitations of chest film examination.
As the reader will appreciate, the utility of plain film examination in the evaluation of adult patients with congenital heart disease is emphasized. This is an important area of rapid growth. This chapter should aid clinicians who encounter such a patient or have an established practice containing many such individuals. Few non–plain film images are included in this chapter; only a few relevant images are included to reinforce mechanisms of radiographic changes in the plain film. One final note on the interpretation of plain chest films: in many cases, one may observe major “obvious” findings that form the basis for an anatomic diagnosis. Further careful analysis of the image, however, often reveals other, more subtle observations that correlate with the major findings. The constellation of all findings allows the observer to assess not only the disease and its severity, but also to assess the confidence the individual has in that diagnosis.
History of Chest Radiography in Cardiac Disease
Wilhelm Conrad Roentgen discovered x-rays on November 8, 1895 (1). Immediately after his discovery, physicians around the world began using this new tool in the evaluation of bone fractures and abnormalities of the gastrointestinal tract. Within months of the discovery, application of the “new photography” to chest examination was reported. Williams (2) reported fluoroscopic examination of a patient with cardiomegaly and described the findings for pericardial effusion. Early examinations were crude and dangerous by today’s standards. For example, Walsh (3) published a text that included a chest film demonstrating the position and dimensions of the heart. The exposure required 20 minutes and was obtained using a 30-inch focus-film (x-ray tube-to-film) distance.
A report of heart size in healthy individuals (4) was published in 1902; by 1909 (5), a review of the normal appearance of the heart, the size and appearance of the heart in patients with aortic and mitral valvular diseases, and a thoracic aortic aneurysm was published. The measurement of the cardiothoracic ratio (the quotient of the transverse diameter of the heart divided by the greatest transverse chest diameter) was introduced by Danzer (6) in 1919. In his paper, he also noted that the normal ratio is probably greater in children. Radiographic demonstration of pericardial calcification (7) and rheumatic valvular calcification (8) were reported in the early 1920s. Evaluation of changes in cardiac size, as determined by computation of the area of the cardiac silhouette in PA chest film examinations of patients with valvular heart disease, myocarditis, systemic hypertension, and hyperthyroidism (9,10) became routine during the latter half of the decade.
Seeking to gain improved insight into the sensitivity of chest film examination for detecting cardiac disease, Ungerleider and Gubner (11) reviewed the PA chest films of 1,460 healthy individuals and published nomograms for normal transverse
diameter and radiographic area of the heart, an indicator of cardiac volume.
diameter and radiographic area of the heart, an indicator of cardiac volume.
The clinical value of plain film examination of the heart was pointed out by Paul Dudley White in an address to the American Roentgen Ray Society in 1929 (12). He emphasized that radiographic examination of the heart provided the most accurate available means of estimating cardiac size and detecting unsuspected pericardial calcification and aortic aneurysms. Furthermore, he praised the technique for its ability to estimate atrial and ventricular chamber enlargement and to provide clinical information in patients too obese or emphysematous for accurate physical examination of the heart. Although there are other imaging modalities, the value of plain chest film examination remains high, and in terms of clinically relevant information obtained, the cost to perform, and patient risk, it remains an integral means of cardiac diagnosis.
The Chest Film Itself: Image Quality
Estimation of the radiographic technique helps the observer to assess the severity of pulmonary vascular changes. A properly penetrated chest examination should allow differentiation of the four basic radiographic densities: air, bone, soft tissue, and fat. Radiographic exposure factors should be chosen so that the resultant examination permits faint visualization of the thoracic spine and the intervertebral disks so that the lung markings behind the heart are clearly visible. Proper radiographic technique allows evaluation of the sharpness of the pulmonary vascular markings and fluid status of the parenchyma. Standard high kilovoltage technique (13,14) radiographs are obtained with a fixed 145 kVp, varying mA between 400 and 600; exposure is controlled by phototimer. Exposure times of between 10 and 60 ms establish an exposure range of between 5 and 30 mA. This method produces clearer visualization of the pulmonary vasculature and better penetration of the mediastinum. However, this technique produces diminished visibility of intrathoracic calcium. Ideally, the lungs should appear gray. Radiographic underexposure (“light films”) generally result in overestimation of the unsharpness of vessels, leading to overinterpretation of pulmonary vascular congestion. Conversely, overpenetration (“dark films”) may produce better visualization of the mediastinal structures, but prevent detailed observation of the pulmonary parenchyma, leading to underestimation of congestion or the belief that pulmonary blood flow is diminished.
Conventional chest film examination of the heart and lungs is performed using a 6-foot PA beam (the upright patient faces the film screen combination), and the radiographic exposure comes from behind (the view is named by the course of the beam, hence PA). Using this method, the x-ray beam is nearly parallel (nondiverging). The ventrally located heart is close to the film screen and is therefore not (or minimally) magnified. Emergency room or intensive care chest radiography is typically performed from in front of the patient who leans against the film cassette. Thus, in this anteroposterior radiograph, the heart is away from the film screen combination, the x-ray beam is not parallel, and therefore diverges. These factors result in magnification of the heart.
If chest radiographs are obtained with the patient upright, we see a reasonable representation of the distribution of interstitial lung water and pulmonary blood flow. When supine, the lower lobe pulmonary vessels lose their gravity dependence, and the upper lobe pulmonary veins become dependent. In the supine chest radiograph, the lungs usually show some degree of pulmonary redistribution, even in an individual with normal cardiac function.
Minor degrees of patient rotation may result in distortion of normal cardiac structures. The degree of film rotation can be judged by evaluation of the trachea, the clavicular heads, and anterior ends of the ribs with the thoracic spine. The trachea is a midline structure, and in a straight PA view, it should be projected over the spinous processes of the cervical and upper thoracic vertebrae. In this view, the lengths of the two clavicles are equal. Furthermore, the distance between the ends of the anterior ribs and the lateral margins of the thoracic spine is equal. Displacement of these landmarks is a sensitive indicator of patient rotation.
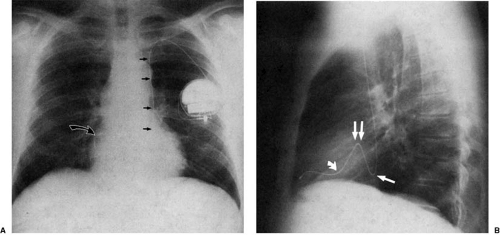
Radiographic signs of previous chest surgery include the obvious placement of midline sternal sutures and mediastinal clips or valvular prostheses as well as more subtle findings, such as the absence of a posterior rib or asymmetry of the intercostal spaces. Surgery in the more distant past may be evident by the appearance of a regenerating rib (Fig. 47.1). More recent surgery may be deduced by asymmetric chest wall edema (Fig. 47.2). In a manner analogous to rotation of the entire chest, scoliosis and chest wall deformity may distort cardiac structures, giving the impression of cardiac rotation or chamber enlargement. When scoliosis is present, changes of pulmonary hypertension and cor pulmonale (15,16) correlate with increasing angle of the scoliosis and patient age (17). Assessment of the size of the heart and state of pulmonary vasculature in these circumstances is difficult. Pectus excavatum (“funnel chest”) is a common deformity consisting of depression of the sternum such that the anterior ribs on each side protrude more anteriorly than the sternum itself. Some investigators have suggested (18) that the primary defect in this lesion is an anomalous position of the heart to the left of midline, leaving the retrosternal space “empty,” allowing the sternum and costal cartilages to fall posteriorly. In the PA projection the heart is rotated and displaced to the left (19). The parasternal soft tissues of the anterior chest wall are viewed in profile rather than en face, increasing their radiographic density, resulting in the appearance of a right middle lobe infiltrate.
The Heart and Great Arteries
Cardiac Position
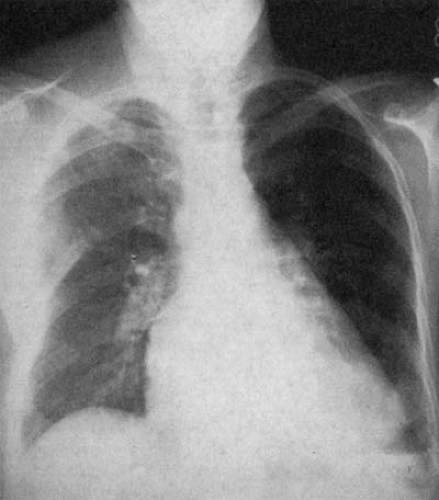
The heart lies just to the left of midline, and the bulk of the ventricular myocardium is in the left chest (Fig. 47.3). The position of the heart within the chest depends on cardiac looping and intrathoracic rotation, as well as the presence of skeletal, pulmonary, and diaphragmatic lesions. In situs solitus, a right-sided heart secondary to a noncardiac abnormality is called dextroposition (Fig. 47.4). This term implies an abnormal position of the heart in the absence of an intrinsic cardiac abnormality. Dextroposition may be caused by abnormalities of the bony thorax (scoliosis, deformities of the sternum or ribs), the lungs (pulmonary agenesis, pneumonectomy, right pneumothorax, chronic volume loss, scimitar syndrome), eventration, or diaphragmatic hernia.
The diagnosis of pneumothorax is made by identification of the visceral pleural line. Only large pneumothoraces produce complete lung collapse and significant mediastinal shift. The roentgenographic density of the ipsilateral collapsing lung changes little until lung volume is greatly reduced (20). In a pneumothorax of any size, shift away from that side is inevitable because pressure in the normal hemithorax remains relatively more negative. Such a circumstance must be differentiated from a tension pneumothorax, in which there is a check valve mechanism, which allows air into the pleural space during inspiration, but prevents its egress during expiration. In tension pneumothorax, one finds inspiratory shift of the heart and mediastinum away from the side of the pneumothorax associated with ipsilateral diaphragmatic depression (Fig. 47.5). Eventration of the diaphragm is a congenital failure of the muscular development of one or both diaphragms (21). Unless occurring in neonates (21,22), this condition does not cause symptoms.
The scimitar syndrome (23,24,25) is an unusual malformation consisting of partial or total anomalous pulmonary venous return of the right lung veins to the inferior vena cava just above or below the diaphragm. It is frequently associated with hypoplasia of the right lung and right pulmonary artery. Frequently, bronchial anomalies and anomalous arterial blood supply to the right lung from the aorta are found (26). PA chest films demonstrate a slight decrease in the size of the right bony thorax and a shift of the heart and mediastinal structures to the right. The characteristic anomalous vein, or scimitar (after the Turkish curved sword), shows a vertical course toward the right cardiophrenic angle, closely paralleling the right atrial border (Fig. 47.6). Depending on the degree of right lung hypoplasia, the scimitar may project through the heart or in the right pericardial area.
Cardiac Situs
Cardiac atrial situs is assumed to be solitus in the presence of thoracoabdominal situs concordance and abdominal situs solitus. Thus, identification of a right-sided liver and left-sided gastric air bubble establishes normal abdominal situs (see Fig. 47.3). Thoracic situs is reliably determined by analysis of the trachea and lungs (27). That is, the morphologic left lung has two lobes (and therefore no minor fissure) and a longer left main bronchus over which the morphologic left pulmonary artery passes proximal to the origin of the left upper lobe bronchus. The morphologic right lung has three lobes (and thus a minor fissure). The right bronchus travels a short course before the origin of the right upper lobe bronchus. The morphologic right pulmonary artery lies anterior and slightly inferior
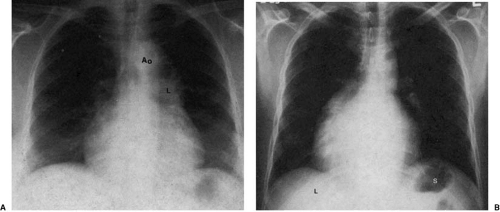
to the right bronchus. Under these circumstances, atrial situs solitus (i.e., morphologic right atrium on the right and morphologic left atrium on the left) can be assumed. The position of the aortic arch or cardiac apex cannot be used to ascertain thoracic situs. Patients with atrial situs solitus may have an isolated right aortic arch or the bulk of their myocardial mass in the right chest (situs solitus with dextrocardia, isolated dextrocardia) in the absence of any other abnormality (Fig. 47.7). Atrial situs inversus is deduced by concordance between inverted abdominal viscera and lung morphology (Fig. 47.8).
to the right bronchus. Under these circumstances, atrial situs solitus (i.e., morphologic right atrium on the right and morphologic left atrium on the left) can be assumed. The position of the aortic arch or cardiac apex cannot be used to ascertain thoracic situs. Patients with atrial situs solitus may have an isolated right aortic arch or the bulk of their myocardial mass in the right chest (situs solitus with dextrocardia, isolated dextrocardia) in the absence of any other abnormality (Fig. 47.7). Atrial situs inversus is deduced by concordance between inverted abdominal viscera and lung morphology (Fig. 47.8).
Aortic Arch and Ascending Aorta
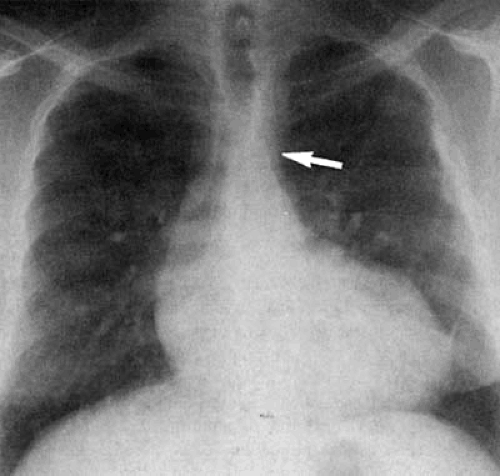
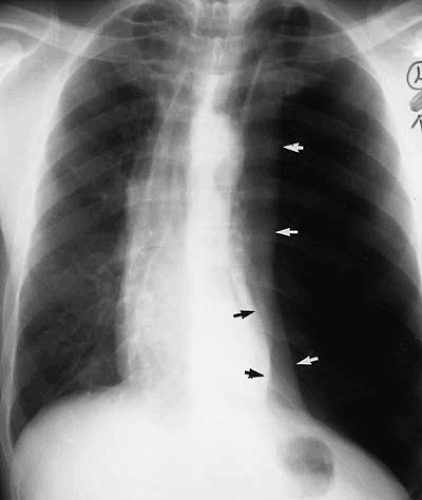
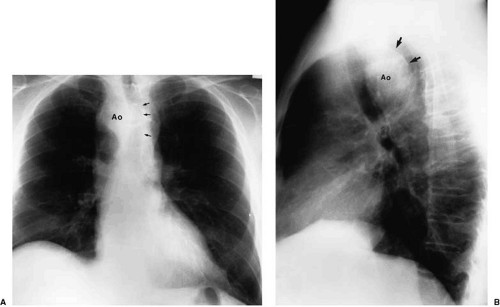
The normal aortic arch passes to the left, displacing the trachea toward the right (see Fig. 47.3). Measurement of the true caliber of the aorta is difficult on plain chest examination because both contours are not visualized in frontal projection. The left border of the descending thoracic aorta is visualized in the PA radiograph, but the right border is not. The contour of the ascending aorta should not extend to the right beyond the vascular pedicle of the right hilum or shadow of the superior vena cava. Enlargement of the ascending aorta is characterized by increased curvature of the middle third of the right heart border or by a forward bulge of the anterior border of the aorta, as viewed on lateral examination (Fig. 47.9). The aortic arch diameter (28) is the sum of the distances measured between the midline and the right-most ascending aortic contour and left-most aortic shadow, measured above the base of the heart. The diameter of the aortic arch may vary in healthy individuals between 1.8 and 3.8 cm, but must not exceed 4.0 cm. However, the ascending aorta, for which there are no “normal” values, is most frequently enlarged in disease.
The proximal descending thoracic aorta may be identified as a straight line seen through the cardiac silhouette running parallel to the spine in continuity with the inferior, medial aspect of the aortic arch. Usually this line is to the left of the spine, but the proximal portion may be right sided in cases of right aortic arch. Unless situs inversus is present, the aorta in
cases of left and right aortic arch enters the abdomen through the left-sided aortic hiatus.
cases of left and right aortic arch enters the abdomen through the left-sided aortic hiatus.
Discrete, rimlike calcification of the aorta and aortic arch is usually a sign of degenerative intimal change, most often resulting from atherosclerosis (Fig. 47.10). The distance between visualized calcification and the outer aortic contour is an estimation of aortic wall thickness; its measurement may be helpful in evaluating acute and chronic changes. If care is taken to consider the effects of supine versus upright examination, and the effects of tangential visualization of the heart and aorta in oblique or anteroposterior versus PA examination, then the distance between intimal calcification and the outer wall of the aorta should be no greater than 10 mm (29). This sign is valuable in the descending aorta but may be of limited value in analysis of the more proximal aortic arch as a result of technical problems described previously.
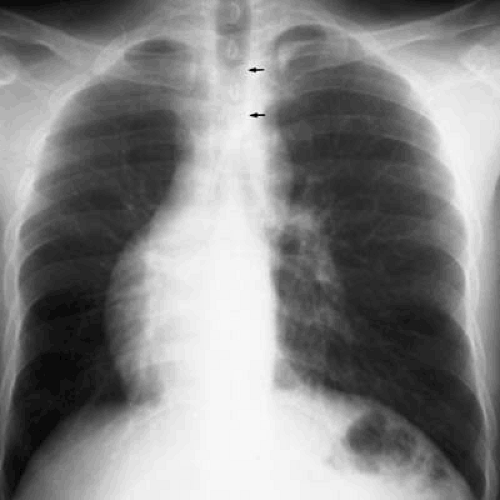 FIGURE 47.7. A 21-year-old man with isolated dextrocardia. The heart and lungs are normal. The cardiac apex is in the right chest. The trachea (arrows) is midline. |
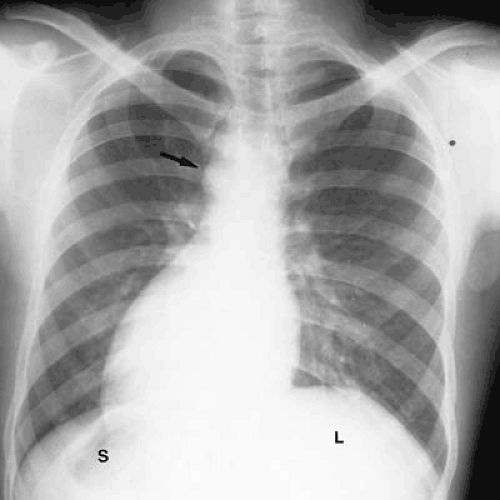 FIGURE 47.8. A 30-year-old man with complete situs inversus. The liver (L) is on the left, and the stomach bubble (S) is on the right. The aortic arch (arrow) and cardiac apex are on the right. |
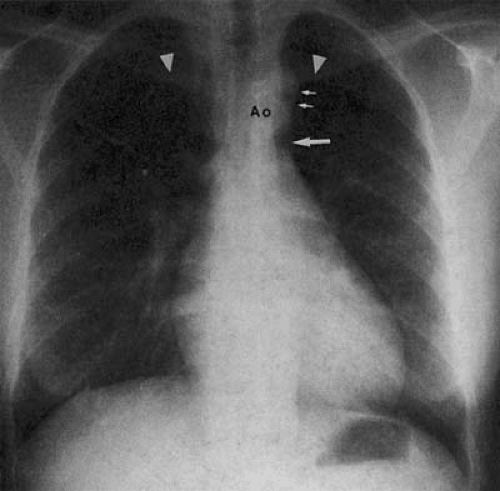
When there is a right aortic arch, the convex shadow of the aortic arch lies on the right side of the superior mediastinum, displacing the tracheal air column to the left (Fig. 47.11). Right aortic arches may be classified (30,31,32) by whether the arch is anterior or posterior to the esophagus. This distinction is important, because the heart is characteristically normal when a posterior arch is present. The presence of an anterior arch is almost universally associated with congenital heart disease.
This distinction is made by evaluation of the lateral chest radiograph. In the posterior right aortic arch, found in approximately 0.1% of the population (33,34), there is a large retroesophageal defect produced by the junction of the posterior right aortic arch with the descending aorta, causing anterior bowing of the tracheal air shadow. This form of right aortic arch is usually asymptomatic and associated with an aberrant left subclavian artery.
This distinction is made by evaluation of the lateral chest radiograph. In the posterior right aortic arch, found in approximately 0.1% of the population (33,34), there is a large retroesophageal defect produced by the junction of the posterior right aortic arch with the descending aorta, causing anterior bowing of the tracheal air shadow. This form of right aortic arch is usually asymptomatic and associated with an aberrant left subclavian artery.
The anterior aortic arch is frequently associated with mirror-image branching. That is, the first branch from the aortic arch is a left-sided innominate trunk, followed by the right common carotid and right subclavian arteries. In this case, there is no retroesophageal component, and thus, no anterior bowing of the tracheal air column (Fig. 47.12). There is a significant association between this form of right aortic arch and congenital heart disease. Between 18% (35) and 34% (33) of patients with tetralogy of Fallot (TOF), 33% (36) and 60% (33) of patients with truncus arteriosus, and 4.9% (24) and 6.7% (33,37) of patients with D-transposition of the great arteries have this form of right aortic arch. Unoperated survival of individuals with these lesions into adulthood is uncommon. However, differentiation among these lesions can be made based on the appearance of the superior mediastinum, the pulmonary artery segment, and the pulmonary vasculature. In patients with TOF, one finds a small pulmonary artery segment and decreased pulmonary vascularity; in D-transposition and persistent truncus arteriosus, the pulmonary artery segment is not found on the left heart border, and there is increased pulmonary vascularity.
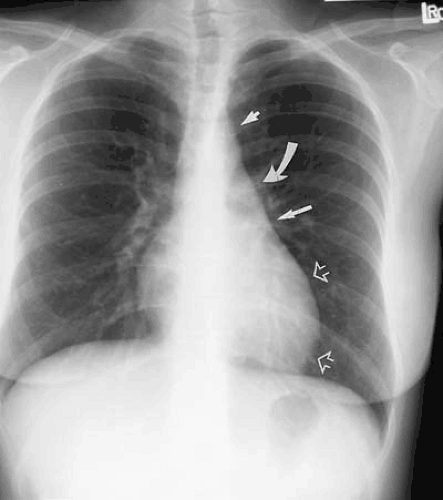
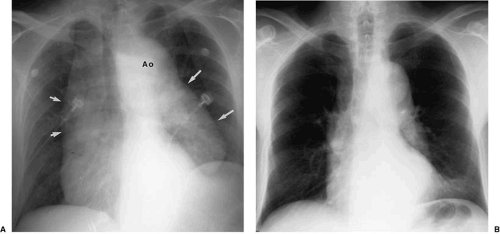
Increase in the caliber of the aortic arch segment may be caused by changes in the aortic wall and are often associated with more generalized changes in the aorta or by localized increase in vessel caliber. The former class of diseases includes atherosclerosis and systemic hypertension. They often result in a prominent-appearing aortic arch, but one not necessarily dilated to pathologic size. Focal atherosclerotic aneurysms of the distal aortic arch or proximal descending aorta may present in association with other localized areas of increase in aortic caliber. Diseases resulting in focal dilatation of the aortic arch include aortic dissection and posttraumatic aortic pseudoaneurysm formation (Fig. 47.13).
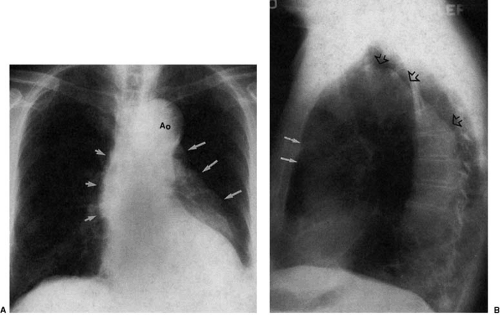
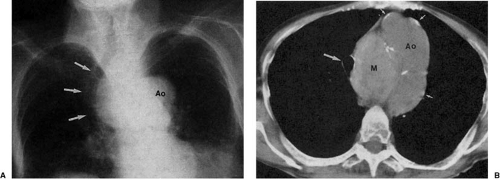
Atherosclerosis is the most common cause of descending aortic aneurysm (38,39,40). These aneurysms are typically located just distal to the origin of the left subclavian artery and appear as fusiform enlargement of the aortic contour (Fig. 47.14). Focal aneurysmal enlargement of the aorta may be difficult to differentiate from diffuse aortic ectasia or from focal mediastinal or pulmonary masses. Most often, however, these aneurysms project into the left chest. Displacement of the distal trachea is most often caused by increased caliber of the proximal portion of the aortic arch or by masses within the superior mediastinum, medially located with respect to the aortic arch (Fig. 47.15).
A high index of suspicion is the most important factor in diagnosing aortic dissection (29). In the appropriate clinical setting, an abnormal chest radiograph lends support to this diagnosis. However, the aortic arch, and in fact the entire chest film examination itself, may appear normal in the presence of an aortic dissection (41). When the aortic arch is involved, one frequently finds widening of the distal portion of the arch, beyond the origin of the left subclavian artery; the aortic arch is abnormal in 80% to 90% of patients with dissection involving the arch (42). Intimal aortic calcification separated by more than 6 (29,43) to 10 (44,45,46) mm from the outer contour of the arch shadow is an excellent indicator of intramural hemorrhage and dissection. One must keep in mind, however, that calcification in a more proximal portion of the aortic arch may be projected medial to the outer contour of the distal arch, and that calcification of mural thrombus or inflammatory thickening of the aortic wall may produce a false-positive diagnosis.
Any subtle or questionable finding becomes significant when a change in appearance from a previous examination is demonstrated (Fig. 47.16).
Any subtle or questionable finding becomes significant when a change in appearance from a previous examination is demonstrated (Fig. 47.16).
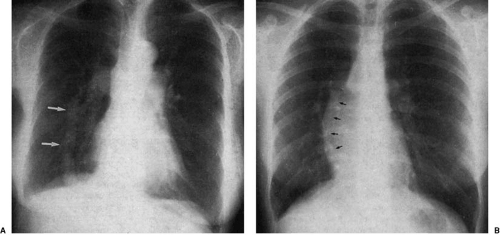
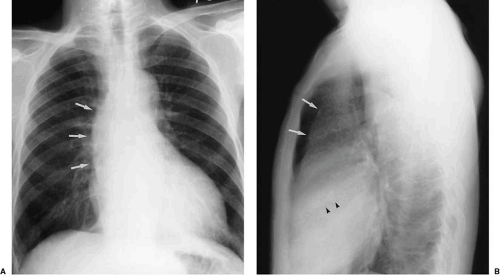
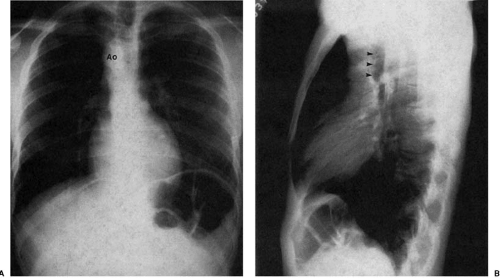
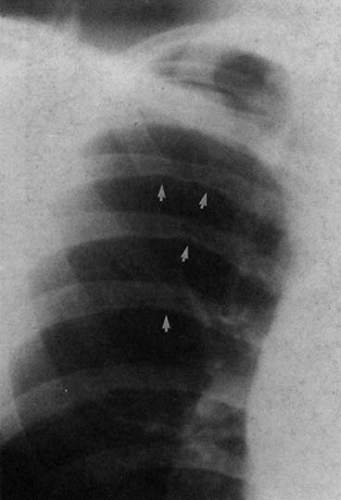
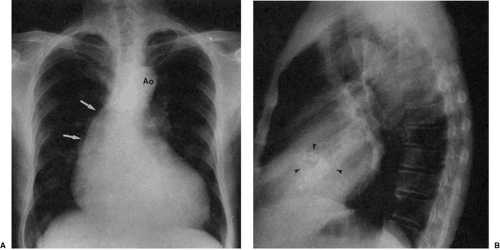
Diminution of the aortic arch segment may be real, as seen in patients with coarctation of the aorta, or apparent, as in the case of individuals with increased pulmonary blood flow in left-to-right shunts. In coarctation of the aorta, there is tubular hypoplasia of the distal aortic arch proximal to the actual area of focal coarctation. The resulting narrow aorta presents as a small, inapparent aortic arch segment (Fig. 47.17). The focal coarctation presents as a concave (to the right) notch in the proximal descending aorta, immediately below the aortic arch. The descending aortic shadow can usually be followed from below in a cephalad direction just to the left of the spine, continuous with the aortic arch shadow. In patients with coarctation, the shadow of the descending aorta is interrupted caudad to its continuity with the aortic arch. Rib notching (Figs. 47.17 and 47.18) is a reliable sign of retrograde collateral
flow to the postcoarctation aorta by dilated intercostal arteries.
flow to the postcoarctation aorta by dilated intercostal arteries.
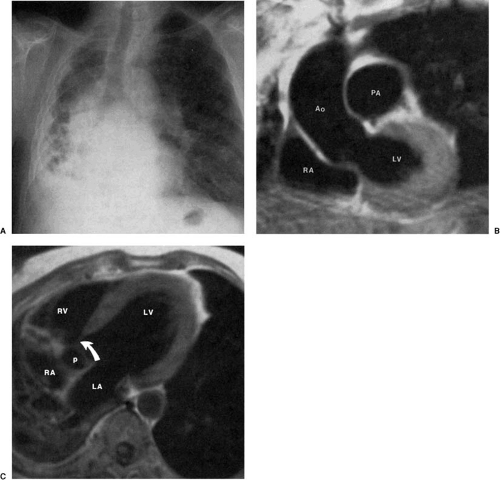
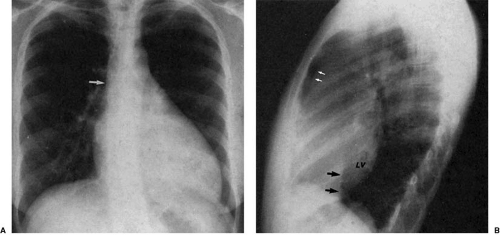
The hearts of patients with corrected transposition of the great arteries have discordant atrioventricular and ventriculoarterial connections. The systemic venous return is as expected, to the right-sided morphologic right atrium. Blood drains across a right-sided mitral valve to the right-sided morphologic left ventricle, which supports a right-sided main pulmonary artery. Pulmonary venous blood returns to the left-sided left atrium, across a left-sided tricuspid valve, and into the left-sided morphologic right ventricle, which supports a left-sided aortic valve and ascending aorta. The left-sided ascending aorta in these patients produces an aortic arch segment that is typically longer and more convex than that found in the normal segment (47,48,49). Furthermore, although the hilar pulmonary arteries may appear normal, the main pulmonary artery
segment of the left heart border is not seen (Fig. 47.19). Mesocardia and dextrocardia are relatively common in corrected transposition of the great arteries. Mirror-image patterns of this anomaly occur in situs inversus (in which the convex upper right cardiac contour is produced by the ascending aorta).
segment of the left heart border is not seen (Fig. 47.19). Mesocardia and dextrocardia are relatively common in corrected transposition of the great arteries. Mirror-image patterns of this anomaly occur in situs inversus (in which the convex upper right cardiac contour is produced by the ascending aorta).
Increase in the prominence of the ascending aortic segment on frontal examination may be a sign of increase in aortic
caliber or tortuosity of the aorta itself. Poststenotic dilatation of the aorta may be found in aortic stenosis (50) (Fig. 47.20). More generalized dilatation of the ascending aorta may be found in aortic insufficiency or in dissection of the aorta. In systemic hypertension or in long-standing atherosclerosis the aorta may become mildly dilated and “meander” through the mediastinum, causing increase in the curvature of the ascending aorta on frontal examination. Whether the aorta is dilated or tortuous, these changes may not always be readily apparent on the frontal examination. If the predominant direction of the
aortic curve is anteriorly into the anterior mediastinum, then the retrosternal space on the lateral examination becomes filled and reveals this abnormality (Fig. 47.21).
caliber or tortuosity of the aorta itself. Poststenotic dilatation of the aorta may be found in aortic stenosis (50) (Fig. 47.20). More generalized dilatation of the ascending aorta may be found in aortic insufficiency or in dissection of the aorta. In systemic hypertension or in long-standing atherosclerosis the aorta may become mildly dilated and “meander” through the mediastinum, causing increase in the curvature of the ascending aorta on frontal examination. Whether the aorta is dilated or tortuous, these changes may not always be readily apparent on the frontal examination. If the predominant direction of the
aortic curve is anteriorly into the anterior mediastinum, then the retrosternal space on the lateral examination becomes filled and reveals this abnormality (Fig. 47.21).
Superior Vena Cava
The superior vena cava is formed by the junction of the left and right brachiocephalic veins dorsal to the right first sternochondral articulation, behind the right border of the sternum. As it descends in the anterior mediastinum, it makes a slight curve convex to the right and dorsalward before emptying into the right atrium at the level of the third costal cartilage ventrally and the seventh thoracic vertebra dorsally. The superior vena cava forms the upper half of the right heart border on the frontal radiograph. The caval shadow is fairly straight and lies parallel to and to the right of the tracheal air column. It extends from the inferior edge of the clavicle to its insertion into the right atrium. At its inferior extension, its insertion into the right atrium, there is a break in the contour of the right heart border, representing the superior aspect of the curve of the right atrium.
The superior vena cava may be displaced to the right by a dilated or tortuous ascending aorta, giving the observer the impression of a widened superior mediastinum. Masses in the superior mediastinum cause extrinsic compression of the cava, resulting in the superior vena cava syndrome. In this circumstance, the cava may actually be displaced medially (to the left) by the mass. More distal caval obstruction (secondary to lymphadenopathy or mediastinal fibrosis) may result in dilatation of the cava and innominate veins, resulting in widening of the mediastinum with prominent curvature of the superior right heart border. Similarly, functional obstruction and the resulting elevation of caval pressure caused by pericardial constriction may present with a dilated superior vena cava. Because there is no obstructing valve in the superior vena cava, elevation of right atrial pressure—such as that found in severe tricuspid regurgitation or right ventricular failure of any etiology—is transmitted back, resulting in dilatation of the azygos vein, and if severe enough, the cava itself.
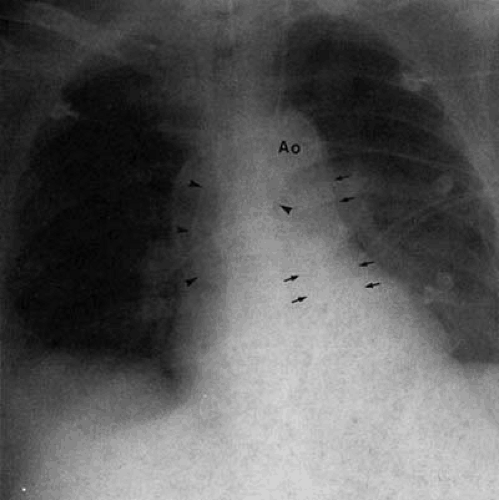
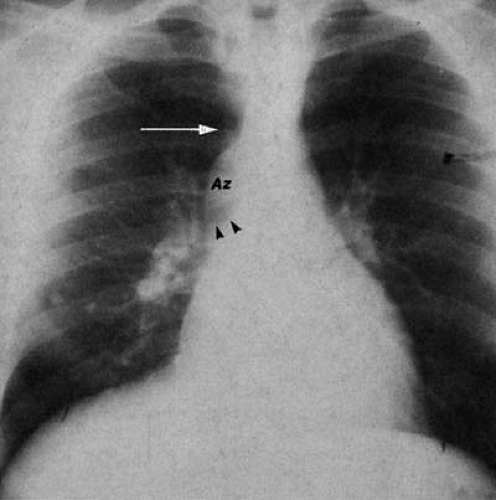
Dilatation of the azygos vein is found in patients with interruption of the inferior vena cava with azygos continuation. PA chest examination reveals a prominent rounded contour adjacent to the right hilum, at the junction of the distal trachea and origin of the right mainstem bronchus (Fig. 47.22). In individuals with isolated lesions, the chest film is otherwise unremarkable. This condition (27,51,52) is frequently associated with polysplenia and left isomerism (bilateral left sidedness),
but may be found as an isolated lesion in adults. Failure of development of the intrahepatic portion of the inferior vena cava results in passage of lower extremity and abdominal venous return to the heart via the dilated azygos vein. Persistence of the left superior vena cava (Fig. 47.23) results from failure of the obliteration of the left anterior cardinal vein. It most frequently drains to the coronary sinus and right atrium, resulting in no hemodynamic insult. In approximately 15% of cases, the right superior vena cava may be absent.
but may be found as an isolated lesion in adults. Failure of development of the intrahepatic portion of the inferior vena cava results in passage of lower extremity and abdominal venous return to the heart via the dilated azygos vein. Persistence of the left superior vena cava (Fig. 47.23) results from failure of the obliteration of the left anterior cardinal vein. It most frequently drains to the coronary sinus and right atrium, resulting in no hemodynamic insult. In approximately 15% of cases, the right superior vena cava may be absent.
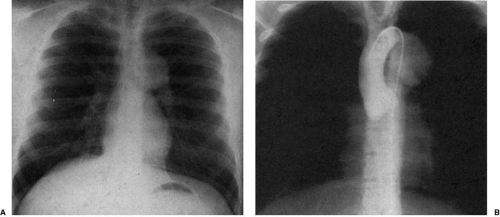
Increased blood flow through the superior vena cava results in its dilatation and increase in the curvature of the superior right cardiomediastinal silhouette. This may be found in children with intracerebral arteriovenous malformations, or in individuals with total anomalous pulmonary venous connection above the heart. In the latter entity, both the left and right superior mediastinal contours are convex; the left contour is dilated by the draining vertical vein and the right by the dilated superior vena cava (Fig. 47.24). These individuals have a characteristic “snowman” configuration of the heart and mediastinum and increased caliber of their pulmonary artery segment and parenchymal pulmonary arteries, evidence of left-to-right shunt.
In individuals with right-sided heart dilatation, the heart rotates (looking from below) in a clockwise manner, bringing the bulk of the cardiac mass into the left chest and causing the superior vena cava to come to lie over the spine, resulting in a narrow-appearing superior mediastinum. This is common in patients with atrial septal defect (ASD) or in patients with pulmonary hypertension and secondary tricuspid regurgitation.
Main and Central Pulmonary Arteries
The main pulmonary artery and pulmonary valve are supported by the right ventricular infundibulum and thus lie cephalad and to the left of the aortic valve. The pulmonary artery segment of the left heart border is composed of the main pulmonary and proximal portion of the left pulmonary artery (see Fig. 47.3). The right pulmonary artery originates from the main pulmonary artery, passing over the roof of the left atrium to enter the right lung. The right hilum is slightly caudad with respect to the left hilum. The main pulmonary artery then passes over the left mainstem bronchus to become the
left pulmonary artery. Because, in PA projection, a portion of the main pulmonary artery segment is derived from the proximal left pulmonary artery, measurement of the caliber of the main pulmonary artery in this view is inaccurate. The main pulmonary artery is best viewed in shallow right anterior oblique projection (Fig. 47.25), which has the effect of rotating the proximal left pulmonary artery posteriorly, away from the cardiac contour. The pulmonary artery appears as a smooth convexity above the upper portion of the right ventricular contour, paralleling the distal trachea and left main bronchus. In individuals with normal pulmonary artery pressure and no left-to-right or right-to-left shunt (left ventricular output equals right ventricular output [Qp:Qs = 1.0]), the size of the pulmonary artery segment should be nearly the same side as the aortic arch segment on the PA radiograph. As the main pulmonary artery ascends and curves to become the left pulmonary artery, it runs away from the sternum to leave a retrosternal clear space behind (Fig. 47.26).
left pulmonary artery. Because, in PA projection, a portion of the main pulmonary artery segment is derived from the proximal left pulmonary artery, measurement of the caliber of the main pulmonary artery in this view is inaccurate. The main pulmonary artery is best viewed in shallow right anterior oblique projection (Fig. 47.25), which has the effect of rotating the proximal left pulmonary artery posteriorly, away from the cardiac contour. The pulmonary artery appears as a smooth convexity above the upper portion of the right ventricular contour, paralleling the distal trachea and left main bronchus. In individuals with normal pulmonary artery pressure and no left-to-right or right-to-left shunt (left ventricular output equals right ventricular output [Qp:Qs = 1.0]), the size of the pulmonary artery segment should be nearly the same side as the aortic arch segment on the PA radiograph. As the main pulmonary artery ascends and curves to become the left pulmonary artery, it runs away from the sternum to leave a retrosternal clear space behind (Fig. 47.26).
 Get Clinical Tree app for offline access 
|