Placement of IVC Filter and Removal of Retrievable Filters
Roan J. Glocker
Marc A. Passman
Introduction
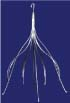
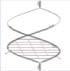
Inferior vena cava filters (IVCFs) represent the latest iteration in a long line of caval interruption techniques including ligation, stapling, partially occlusive clips, and surgical plication. Now, there are many FDA-approved IVCFs available, each with slightly different features, but all designed to prevent venous thromboembolism (VTE) by trapping emboli from lower extremity veins before entering the pulmonary circulation (Table 9.1).
For the majority of patients with VTE, whether presenting as deep venous thrombosis (DVT) or pulmonary embolism (PE), therapeutic anticoagulation is the standard therapy based on evidence-based guidelines. For patients with recurrent PE despite therapeutic anticoagulation, inability to achieve appropriate anticoagulation, and hemorrhagic complication or contraindications to therapeutic anticoagulation, placement of an IVCF is indicated (Table 9.2). Relative indications for IVCF placement should be considered on a case-by-case basis and include free-floating thrombus in the inferior vena cava (IVC); poor compliance with anticoagulation; limited pulmonary reserve; as an adjunct during iliocaval thrombolysis; or as prophylaxis in critically ill patients with significant burden of injury, severely compromised mobility, and risk of bleeding preventing therapeutic anticoagulation. IVCF placement is contraindicated in patients who have congenital absence of the IVC, and special considerations are made for patients with congenital IVC anomalies such as duplicated IVC. Suprarenal IVCF may be a consideration for venous thrombosis of the infrarenal IVC associated with recurring PE or in patients with significant compression of the infrarenal IVC such as by a malignancy or a gravid uterus consideration.
Table 9.1 Images and Design Characteristics of Currently Available FDA-approved Inferior Vena Cava Filters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
With the increasing use of upper extremity lines, central lines, and implantable cardiac devices upper extremity DVT is becoming a more common entity. As such, PE from an upper extremity source is a possibility. For those patients who are unable to receive anticoagulation, placement of a superior vena cava (SVC) filter may be warranted, although this is outside the indications for use (IFUs) for current IVCFs.
Once the clinical decision is made to proceed with IVCF placement, the choice of whether to place a permanent or retrievable IVCF must be made. The indication for IVCF placement is considered with respect to the duration of need. For patients with recoverable injuries or illness, or those who need VTE prophylaxis for a defined time period, retrievable IVCFs may be favored. Those with nonrecoverable injuries, extended duration of illness, or without a defined IVCF retrieval timeline, should have a permanent IVCF placed. Although all retrievable devices have a permanent IFU, there is evidence that long-term complications with retrievable devices left in for extended times are greater than those for IVCFs designed for permanent use only. There is also
increasing need to ensure that patients with retrievable IVCFs return for retrieval consideration. Recent evidence suggests that retrieval attempts made closer to the time of placement are more likely to be successful compared with those attempted after a longer IVCF dwell time. Based on current FDA guidelines, retrievable IVCFs should be removed as soon as clinically allowable once VTE risk has diminished or bleeding risk has decreased enough so that anticoagulation can be used.
increasing need to ensure that patients with retrievable IVCFs return for retrieval consideration. Recent evidence suggests that retrieval attempts made closer to the time of placement are more likely to be successful compared with those attempted after a longer IVCF dwell time. Based on current FDA guidelines, retrievable IVCFs should be removed as soon as clinically allowable once VTE risk has diminished or bleeding risk has decreased enough so that anticoagulation can be used.
Table 9.2 Indications and Contraindications to IVCF Placement | ||||||
|---|---|---|---|---|---|---|
|
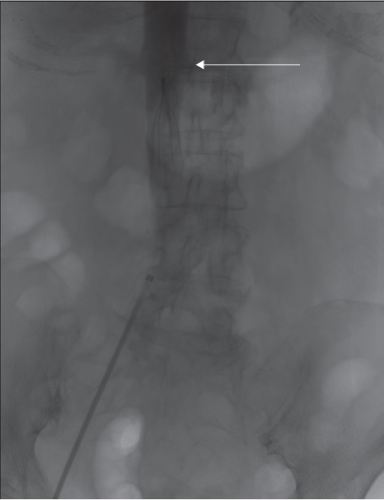
For IVCF placement, preoperative evaluation includes assessing bleeding risk with coagulation studies, and suitable percutaneous access sites free of DVT or pre-existing catheters. A preprocedure review of available images should be performed to ensure that the patient has suitable anatomy for IVCF placement and that the vena cava is of a suitable size to meet the IFUs for the intended device. Preprocedure venous duplex ultrasound should be reviewed for presence and location of DVT, and to confirm an access site free of thrombus. Additional review of any available CT scans is helpful to determine possible venous anomalies, suitability for bedside techniques, and IVCF selection.
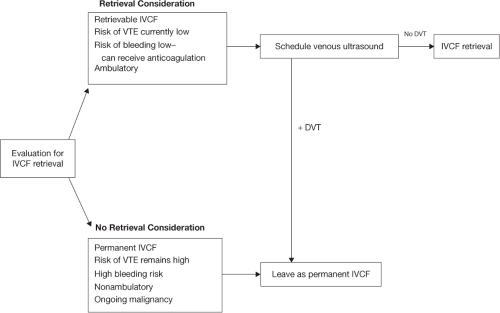
For IVCF retrieval, a preoperative clinical decision algorithm is shown in Figure 9.1. When planning for IVCF retrieval, a careful history is performed to ensure that the patient has a low current risk of VTE, can receive anticoagulation if there is still VTE risk, is no longer immobile, and confirming that no further operations that would require avoidance of anticoagulation are needed in the immediate future. A review of a recent abdominal radiograph is necessary to confirm IVCF type and ensure that there has not been any IVCF migration, penetration, or fracture. A venous ultrasound should also be performed to ensure that there is no ongoing DVT concerns and to confirm patency of potential access sites. If there are ongoing VTE risk concerns and bleeding risk remains high, IVCF should be left as a permanent device.
General Considerations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree