Proximal Debranching Using the Ascending Aorta for Bypasses to Carotid, Innominate, and Subclavian Arteries with Endovascular Repair of Thoracic and Arch Aneurysms
Mark A. Farber
Jason Crowner
Advancements in medicine have resulted in a steady increase in life expectancy. In conjunction with this increase in age, there have been improvements in medical care and capabilities of diagnostic imaging, thereby enhancing the detection of various lesions including arch and thoracic aortic pathology. These pathologies include a combination or isolated occurrence of arch or thoracic aneurysms, aortic dissections, penetrating atherosclerotic ulcers, and intramural hematomas. With respect to aneurysmal disease, ascending aneurysms are the most prevalent (40%), with aneurysms of the descending thoracic aorta accounting for 35%, arch aneurysms 15%, and thoracoabdominal 10%. The population in which these pathologies are found can often be elderly or frail, and it is often difficult to determine the best method of repair. It should be noted that not only can these patients be older, but also they are more likely to have a larger number of comorbidities including, but not limited to, chronic obstructive pulmonary disease, heart-related issues including arrhythmias and failure, renal insufficiency, diabetes, and cerebral vascular disease. Czerny has demonstrated that each of these adds significant risk to the treatment of lesions in the ascending aorta and the aortic arch.
Open aortic arch operations have been shown to be associated with a significant incidence of in-hospital mortality of up to 20% and a stroke rate of up to 12% as demonstrated by Coselli et al. Isolated thoracic aortic aneurysms have been shown to undergo progressive expansion leading to death in up to 45% to 85% if untreated within 5 years as demonstrated (Juvonen et al.). Accepted indications for treatment are listed as follows:
Symptomatic aneurysms of the aortic arch and descending thoracic aorta regardless of size,
Asymptomatic aneurysmal disease >5.5 cm,
Acute and some chronic type A dissections,
Acute complicated and some chronic type B dissections,
Intramural hematomas present in the arch, and
Penetrating aortic ulcers.
The traditional open operative intervention involves complete arch replacement for arch aneurysms with an interposition graft. This typically requires a median sternotomy, cardiopulmonary bypass, and in some cases hypothermic arrest. While these operations have been performed for many years, their outcomes are associated with significant risks. The Mayo clinic group reported that open aortic arch repair carries a 16.8% mortality and a stroke rate of 9.5%. Coselli et al. have published their results with an in-hospital mortality of up to 20% and stroke rate of up to 12% in the elderly population. Other associated risks include possible respiratory complications, paraplegia, and perioperative cardiac events. Given the increased risk profile of the patients undergoing these operations, there has been a significant interest in using newer endovascular technologies in an effort to reduce the morbidity and mortality of repair associated with the aforementioned lesions. With the continued maturation of endovascular devices, and surgeons becoming more adept with their use, hybrid and complete endovascular approaches are being employed more often. In contrast to open repair, the purely endovascular approach limits the physiologic stress, which in other aortic pathology treatment has been associated with lower major adverse events. Slowly, we are moving toward a complete endovascular approach to repair these lesions as new developments in device design progress. Until devices become more universally available, the hybrid approach, involving great vessel debranching followed by endovascular stent graft exclusion, has gained wider use. While hybrid repair of TAAA has been met with mixed results, those for arch aneurysms appear to have improved outcomes compared to traditional open repair.
Degenerative aneurysms of the arch and thoracic aorta are the most common disease process requiring repair. The natural history of aneurismal disease has been discussed elsewhere in this chapter. Briefly, aneurysm formation is often secondary to cystic medial degeneration. This process leads to degeneration of the vessel wall that results in aneurysm formation or growth ≥1.5 times the normal diameter. The indications for repair of an arch or thoracic aneurysm not only involve the size but also the growth rate. The size necessitating operative repair is generally accepted as being approximately 5.5 cm based on several previously published articles. The decision for intervention is then adjusted in accordance with the patients’ individual risk factors, such that when the risk of repair is less than or equal to the risk of rupture, the repair is encouraged. Factors that influence these numbers not only involve absolute diameter but also the growth rate, and whether the patient is symptomatic. Growth of an aneurysm greater than 0.5 cm per year is labeled as rapidly expanding and generally necessitates operative intervention.
The presence of a symptomatic aneurysm is an absolute indication for operative repair unless the patient is a prohibitive risk. Symptomatic aneurysms usually present with a constellation of symptoms including abdominal pain, back pain, generalized discomfort, and occasionally associated nausea/vomiting. Radiologic assessment should be done immediately upon patient evaluation, if no contraindication; a contrasted CTA is the imaging modality of choice. Imaging findings can be discreet but worrisome aspects include focal discontinuity of intimal calcifications, high-attenuating crescent
sign which represents acute hematoma within either the mural thrombus or aneurismal wall, the presence of periaortic stranding, and draping of the posterior aorta over the vertebrae. Pending or contained ruptures should be managed in an expedited fashion as progression to full rupture is variable and is almost universally fatal. The presence of retroperitoneal hemothorax, hemomediastinum, and extravasation of contrast are pathognomonic for rupture. This patient population generally presents with similar symptoms and associated hypotension. When a free rupture is present, this indicates a surgical emergency requiring immediate intervention and is associated with a high morbidity and mortality.
sign which represents acute hematoma within either the mural thrombus or aneurismal wall, the presence of periaortic stranding, and draping of the posterior aorta over the vertebrae. Pending or contained ruptures should be managed in an expedited fashion as progression to full rupture is variable and is almost universally fatal. The presence of retroperitoneal hemothorax, hemomediastinum, and extravasation of contrast are pathognomonic for rupture. This patient population generally presents with similar symptoms and associated hypotension. When a free rupture is present, this indicates a surgical emergency requiring immediate intervention and is associated with a high morbidity and mortality.
The principal contraindications to the performance of a hybrid repair are dependent on patients’ comorbidities and overall health status. Preoperative evaluation is vital to patient selection and ability to safely perform the operation with a reasonable morbidity and mortality. Previous open-heart operations and radiated fields make dissection planes almost indiscernible and greatly limit the ability to safely expose the great vessels. Evaluation of anatomy demonstrating a highly calcified aorta is a contraindication to clamping of the aorta for performance of the ascending bypass. The bypass can also be complicated or contradicted in the setting of major occlusive disease involving the supra-aortic vessels or aorto-iliac segments. In addition, the diameter and length of the intended landing zone must be critically assessed. If too large or insufficient length available for creating a proximal seal, then the hybrid approach should be abandoned in favor of open surgical reconstruction. Endograft placement can also be limited by ability to traverse diseased aorto-iliac segments, in such instances the need for antegrade endograft deployment should be considered.
Patient selection and proper preprocedural planning is an important aspect of surgical preparation for successful repair of arch and thoracic aneurysms. The initial workup of a patient with an arch or thoracic aneurysm prior to operative repair should include the following:
Axial imaging (computed tomography or magnetic resonance imaging) with and without contrast in conjunction with centerline imaging
Ultrasound evaluation of the carotid and vertebral arteries
Assessment of the circle of Willis
Routine laboratory values
Pulmonary function test
Chest x-ray
Electrocardiogram (EKG)
Echocardiography
Centerline imaging allows review of the anatomy along with the ability to evaluate for significant occlusive disease involving the supra-aortic branches or aorto-iliac segments. Evaluation of the iliac vessels and aortic bifurcation will be beneficial in planning the endovascular aspect of the procedure. Duplex evaluation of the carotid and cerebral vessels excludes hemodynamically significant stenoses or occlusions, and addresses perfusion in the vertebral arteries. This information is not only critical in defining the extent of disease, but is paramount in facilitating the operative plan for hybrid approaches, and includes hemodynamic assessment of the cerebral and upper extremity circulation. While the focus of imaging is centered upon the aorta and its branches, one must also not forget to evaluate the intracranial circulation for completeness of the circle of Willis, as well as identification of aberrant anatomy which can lead to significant complications if not recognized preoperatively.
The endovascular planning relies heavily on preoperative imaging with centerline evaluation. The treatment area length and diameter should be closely reviewed, as endograft selection is dependent on these factors. Proximal and distal seal zone diameters approximately 2 cm from diseased portion need to be reviewed when selecting graft size. The endograft diameter chosen should be approximately 20% greater than the proximal and distal seal zone diameters to ensure adequate seal but should follow specific device guidelines.
Before proceeding to the operating room, risk stratification with assessment of cardiac and pulmonary function should be established to assist with operative decision-making and management. Patients may need cardiac clearance, which often includes chemical or traditional stress tests and echocardiography. Lumbar drains can be placed prior to the operation or the day of operation. This should be decided prior to the operative date and also communicated to the patient regarding the risks and benefits. The use of a lumbar drain should be considered in patients requiring long segments of aorta to be covered with an endograft or those who have had previous endograft placement. After complete preoperative assessment, the risks and benefits should be thoroughly discussed with the patient, along with a realistic approach to the outcomes and expectations including the follow-up recommendations and potential for secondary interventions.
As with all endovascular techniques, stent-graft repair of aneurysms requires anatomically appropriate necks or landing zones for proper graft fixation to allow for exclusion of the lesions. The largest available, FDA-approved stent graft has a diameter of 46 mm indicated for treatment of a neck aortic diameter of no more than 42 mm. In addition, the length of the landing zone necessary to achieve adequate proximal fixation and seal, in general, must be no less than 2 cm, possess parallel walls, and be relatively free of disease. This becomes extremely difficult in the ascending, arch, and proximal thoracic aorta due to its curvature. In our experience, it is therefore necessary to target a significantly greater length to achieve proper exclusion of pathologies except in rare circumstances. To achieve this length, critically important arteries often require coverage necessitating debranching to maintain perfusion to critical structures. The approach of short neck fixation to avoid great vessel reconstruction should be avoided; experience has demonstrated that approaching lesions in this fashion increases the need for secondary operations which are often more difficult than the primary procedure and only serve to increase the overall risks to the patient.
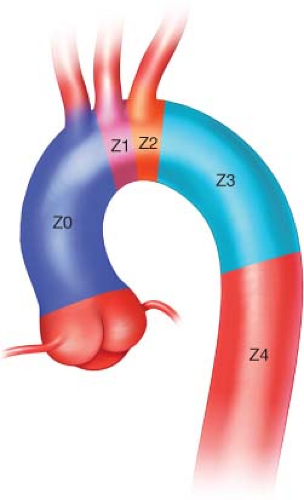
For evaluation and planning the thoracic aorta is divided into four zones (Fig. 1.1). Landing zone 0 involves the ascending aorta to the innominate artery, zone 1 involves
the aortic arch distal the innominate and including the left common carotid artery (CCA), zone 2 involves the aortic arch distal to the left CCA and including left subclavian artery (SCA), zone 3 involves the proximal descending thoracic aorta distal to the left SCA, and zone 4 involves the mid descending thoracic aorta. The degree of debranching required for repair is dictated by the location of suitable proximal landing zone. The next few sections will discuss using a hybrid approach when zones 0 and 1 are involved.
the aortic arch distal the innominate and including the left common carotid artery (CCA), zone 2 involves the aortic arch distal to the left CCA and including left subclavian artery (SCA), zone 3 involves the proximal descending thoracic aorta distal to the left SCA, and zone 4 involves the mid descending thoracic aorta. The degree of debranching required for repair is dictated by the location of suitable proximal landing zone. The next few sections will discuss using a hybrid approach when zones 0 and 1 are involved.