Physiologic and Metabolic Study of Pulmonary Disorders Using Conventional Imaging Techniques and Positron Emission Tomography
INTRODUCTION
Since the mid-1950s, the use of radiopharmaceuticals has made it possible to assess a variety of pulmonary disorders. In 1955, 133Xe was introduced for the study of regional pulmonary ventilation.1 Shortly thereafter, it became possible to evaluate regional pulmonary blood flow using inhaled carbon dioxide containing radioactive 15O2 or intravenous injection of 133Xe dissolved in saline solution.3 In 1964, intravenous injection of 131I-macroaggregated albumin made it feasible to obtain perfusion scans of the lungs.4 Although these techniques rapidly gained wide acceptance as tests of regional abnormalities in ventilation and pulmonary blood flow, the main practical application has been in the diagnostic evaluation of patients with suspected pulmonary embolism (PE). Increasingly, the role of nuclear medicine in respiratory medicine has been expanded to include disorders such as preoperative assessment of lung function, inflammatory lung disease, and lung cancer. The more widespread availability of positron emission tomography (PET) and integrated PET/CT (computed tomography) has provided powerful tools to aid in the diagnosis, staging, and management of patients with lung cancer.
RADIOPHARMACEUTICALS AND TECHNIQUES IN VENTILATION–PERFUSION LUNG SCANNING
Radiopharmaceuticals commonly utilized in both perfusion and ventilation studies, as well as the techniques employed are discussed in subsequent sections.
 PERFUSION AGENTS AND TECHNIQUES
PERFUSION AGENTS AND TECHNIQUES
Clinical application of perfusion lung scanning was first described in 1964, when iodine 131–labeled macroaggregates of albumin was utilized in the evaluation of pulmonary perfusion.4 Currently, the two agents used for pulmonary perfusion imaging are technetium 99m–labeled human albumin microspheres (99mTc HAM) and macroaggregated albumin (99mTc MAA). 99mTc MAA particles range in size from 10 to 150 μm; more than 90% of injected particles measure between 10 and 90 μm. 99mTc HAM particles are relatively uniform in size and range between 35 and 60 μm. However, 99mTc MAA is considered the agent of choice for routine perfusion lung scanning because of its availability, short residence time in the lungs, and relatively low cost.
Radiolabeled particles are injected intravenously while the patient is in the supine position, thereby limiting the effect of gravity on regional pulmonary arterial blood flow. Following the administration of 99mTc MAA, particles mix uniformly with venous blood flowing to the heart; the particles lodge in precapillary arterioles of the lungs, obstructing approximately 0.1% of their total number. The usual administered dose of radioactivity is between 74 and 185 MBq (2–5 mCi) (typically, 200,000 to 500,000 particles are injected during clinical perfusion scan). The usual pediatric-administered activity is 0.5 to 2.0 MBq/kg (20–80 mCi/kg), with a minimum of 7 to 8 MBq (approximately 200 mCi).
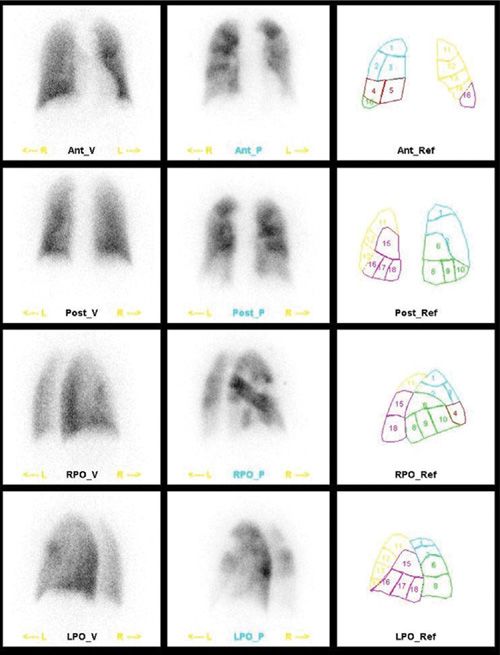
The blockage of pulmonary precapillary arterioles by 99mTc MAA is transient; the biologic half-life in the lung ranges between 2 and 8 hours. The physical half-life of 99mTc is 6 hours. The MAA particles are cleared by enzymatic hydrolysis, forming smaller particles that are phagocytized by reticuloendothelial cells. In pediatric patients and patients with suspected or known right-to-left shunts, severe pulmonary hypertension, poor respiratory function, pregnancy, prior pneumonectomy, or a single lung transplant, the number of particles injected should be reduced. In infants and children, the use of low number of particles can be calculated based on weight.5 The distribution of particles in the lungs is proportional to regional pulmonary blood flow at the time of injection.6 A routine perfusion scan should include at least six views of the lungs: Anterior, posterior, right and left lateral, and right and left posterior oblique views, using large field of view high-resolution gamma camera. Right and left anterior oblique views may be helpful in selected cases. In spite of imaging in multiple projections, the perfusion scan may underestimate perfusion abnormalities. A solitary segmental perfusion defect within the medial basal segment of the right lower lobe is completely surrounded by normal lung. Consequently, a perfusion defect in this segment will not be detected on planar perfusion imaging.
Perfusion lung scans are routinely utilized to examine patients with suspected PE. Unfortunately, perfusion imaging is sensitive, but not specific, for diagnosing PE. Virtually all lung diseases (including tumors, infections, asthma, and chronic obstructive pulmonary disease [COPD]) may cause decreased pulmonary arterial blood flow in the affected lung zones. Therefore, combined use of perfusion and ventilation studies improves the diagnostic specificity of lung scanning for PE (Fig. 32-1). PE almost always causes abnormal perfusion, while ventilation is preserved (mismatched defects) (Fig. 32-2). In contrast, in parenchymal pulmonary disorders, decreased ventilation and perfusion are noted in the same lung region (matched defects). Conditions in which the ventilation abnormality may appear larger than the perfusion abnormality (reverse mismatch), indicating a functional right-to-left shunt, include airway obstruction, mucus plug, atelectasis, and pneumonia.7 Reverse mismatch may be exacerbated in patients on positive end-expiratory pressure (PEEP)8 as PEEP is less efficiently transmitted to obstructed, well-perfused areas, in patients with metabolic alkalosis,9 or in patients treated with inhaled albuterol.
Figure 32-1 Normal ventilation–perfusion lung scanning. Ventilation scan using 99mTc Technegas® aerosol (left column): Uniform distribution of the aerosol throughout both lungs. Perfusion scan using 99mTc MAA (middle column): Uniform distribution of particles throughout both lungs.
Figure 32-2 High probability scan for pulmonary embolism. Ventilation scan using 99mTc Technegas® aerosol (left column) is within normal limits. Perfusion scan using 99mTc MAA (middle column) shows large segmental defects in both lungs. This combination of findings (mismatch) is consistent with pulmonary embolism.
 VENTILATION AGENTS AND TECHNIQUES
VENTILATION AGENTS AND TECHNIQUES
Historically, 133Xe has been the agent used to determine regional ventilation.1,10 133Xe has a physical half-life of 5.24 days and a low gamma energy emission of 81 keV compared to 140 keV for 99mTc. The examination is somewhat laborious and encompasses three phases. Thus, other tracers, such as 81mKr, and, the 99mTc-labeled aerosols – Technegas and Pertechnegas – are now replacing it.
81mKr is a noble gas that has a very short physical half-life (13 seconds). Therefore, images acquired using this agent reveal ventilation to major airway systems only. However, the short physical half-life of 81mKr allows generation of lung images in multiple projections that can be matched with perfusion images. 81mKr is produced from a rubidium-81 generator. The parent radionuclide has a physical half-life of 4.7 hours, which limits the useful lifetime of the generator to only 1 day. Imaging with 81mKr is generally performed following the perfusion scan due to its higher energy than 99mTc.
A radioaerosol is produced by nebulizing the radiopharmaceutical into a fine mist, which is subsequently inhaled. 99mTc-labeled aerosol studies can be performed following the inhalation of several preparations; 99mTc DTPA (diethylene triamine penta-acetic acid) is the most popular and commonly used worldwide. The advantages of 99mTc aerosols are that they are widely available, inexpensive, and have a 140-keV energy photopeak, which is ideal for gamma camera imaging. One limitation of this agent is its relatively rapid absorption across the pulmonary capillaries into the blood in the presence of inflammation, including that caused by smoking (typical 99mTc DTPA aerosol biologic half-life is about 80 minutes compared to ∼20 to 30 minutes in smokers).11,12 Since 99mTc DTPA aerosol is cleared from the alveoli by transepithelial diffusion, the clearance rate may be used as an index of alveolar–epithelial membrane integrity.11
99mTc-labeled radioaerosols have particles between 0.5 and 2 μm in size and are produced by utilizing commercially available nebulizers.13 The patient generally breathes from the nebulizer (with oxygen at 8 to 10 L/min) for 3 to 5 minutes or until 37 MBq (1 mCi) of radioactivity is deposited in the lungs. Only 2% to 10% of administered radioactivity goes to the lungs. The regional distribution of radioactivity in the lungs is proportional to local ventilation. 99mTc-labeled radioaerosol studies are generally performed before perfusion imaging. Although less preferred, the ventilation study can be performed following the perfusion scan, but the dose placed in the nebulizer should be increased to at least 1665 MBq (45 mCi), instead of the usually used 1110 MBq (30 mCi) to overwhelm the activity present in the lungs from 99mTc MAA.
The lungs are imaged in multiple projections, which correspond to those obtained during the subsequent perfusion study. Ventilation studies using 99mTc-labeled radioaerosols require minimal patient cooperation and can be performed at the bedside and on patients who are on ventilators. Disadvantages of 99mTc-labeled radioaerosols include the central deposition of radioactivity in patients on PEEP, patients with COPD or airway obstruction, and the need to dispose of the substantial unused amount of radioactivity that is deposited in the nebulizer. Indeed, the central deposition of 99mTc-labeled radioaerosol in patients with COPD is a major drawback to the use of aerosol agents, and newer agents have been developed to overcome this deficiency, including 99mTc Technegas and 99mTc Pertechnegas.14
LUNG SCANNING IN THE DIAGNOSIS OF ACUTE PULMONARY EMBOLISM
PE is a common and potentially fatal disorder for which treatment is highly effective in decreasing mortality and morbidity if initiated soon after the event.15 The accurate and expeditious diagnosis of acute PE can be difficult because of the nonspecificity of clinical, laboratory, and radiographic findings. Despite diagnostic advances, delays in diagnosis of PE are still common and represent an important clinical issue.16
Venous thromboembolism (VTE) and PE are no longer considered a one-time only event, as they have a high recurrence and mortality rate.17 Although the incidence of PE has not significantly changed over the past three decades,18 the overall mortality rate from PE has decreased substantially (by roughly 30% from 1998 to 2009),19 which has been attributed to better detection and treatment of deep venous thrombosis (DVT), risk factor modification, and improvement in PE diagnostic tests.19–21 Although prompt anticoagulation therapy remains the cornerstone of PE treatment, as it is effective and reduces mortality, it is not without risks, including hemorrhagic complications.22 Therefore, accurate diagnosis of PE is essential, not only to prevent death from recurrent embolism but also to avoid complications related to unnecessary anticoagulant therapy.
Ventilation–perfusion (V/Q) lung imaging has been shown to be a safe, noninvasive technique in evaluating regional pulmonary function undertaken for a variety of purposes. The technique has been widely used in the assessment of patients with suspected PE.
The first major study that utilized perfusion lung scanning as a screening test for the diagnosis of PE was the Urokinase Pulmonary Embolism Trial (UPET).23 In more than 90% of patients enrolled in the trial, perfusion lung scanning was performed following intravenous administration of 131I-labeled MAA. Lung imaging was carried out using rectilinear scanners; ventilation studies were not performed. Despite utilizing a suboptimal radiopharmaceutical and imaging equipment, the UPET study established perfusion lung scanning as an effective technique in both screening for PE and assessing restoration of pulmonary blood flow following an embolic event. Approximately 75% to 80% of perfusion defects resolved by 3 months; those that did not, persisted after 1 year, indicating that most patients with acute PE either completely lyse the thrombi or partially recanalize the pulmonary artery.
Data from prospective, large, outcome-based studies have reported on the important diagnostic information provided by V/Q scanning in patients suspected of having acute PE when coupled with clinical assessment and noninvasive leg testing.24,25 In patients with suspected PE who had a non–high probability or nondiagnostic V/Q scan, normal cardiorespiratory reserve, and negative serial noninvasive leg tests for proximal venous thrombosis (as determined by serial impedance plethysmography [IPG]), PE at 3 months of follow-up occurred in only 0.6% of cases while the patients were not on anticoagulation.24 Among patients with suspected PE, but who had a low probability clinical assessment and negative serial noninvasive leg tests, as well as a nondiagnostic V/Q scan, PE at 3 months follow-up occurred in 0.5% of patients.25 When the probability for clinical assessment was intermediate, PE occurred in only 0.4% of patients.25
These studies demonstrated that anticoagulation could be safely withheld in patients with adequate cardiorespiratory reserve who did not have high probability V/Q scans or proximal venous thrombosis, since the incidence of recurrent PE is very low. Unfortunately, the criteria used in the studies to categorize the probability of PE (“normal,” “nondiagnostic,” or “high”) were different than those used in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED), and direct comparison is not possible. In the Prospective Investigative Study of Acute Pulmonary Embolism Diagnosis (PISA-PED),26 which utilized perfusion scanning alone in conjunction with the chest radiograph, the sensitivity and specificity of scintigraphy were 92% and 87%, respectively. The prevalence of PE was high (39%). When considered in conjunction with clinical assessment of the likelihood of PE (“very likely,” “possible,” or “unlikely”), the positive predictive value (PPV) of a perfusion scan was 99%; the combination of a near-normal or abnormal perfusion scan without segmental defects and low clinical likelihood of PE had a negative predictive value (NPV) of 97%. Using standardized clinical assessment and perfusion lung scanning, the authors were able to accurately diagnose or exclude PE (PPV, 96%; NPV, 98%). CT angiography (CTA) was required in only a minority of cases having discordant clinical and scintigraphic findings.
 PROSPECTIVE INVESTIGATION OF PULMONARY EMBOLISM DIAGNOSIS STUDY
PROSPECTIVE INVESTIGATION OF PULMONARY EMBOLISM DIAGNOSIS STUDY
To date, the most comprehensive prospective investigation addressing the role of V/Q scanning in the diagnosis of PE has been the PIOPED study. This multi-institutional study was designed to evaluate the efficacy of various conventional methods for diagnosing acute PE. In particular, PIOPED focused on the sensitivity and specificity of lung scans in the diagnosis of acute PE. Although the clinical diagnosis of PE is not definitive, results from PIOPED emphasize the importance of incorporating clinical assessment in evaluating patients suspected of having acute PE. As expected, combining clinical assessment with lung scan interpretation improves diagnostic accuracy of the imaging technique.
Ninety-two percent of patients with PE in PIOPED had at least one of the following risk factors: Immobilization and recent surgery (two most common risks), underlying malignancy, history of DVT or PE, estrogen use, or pre-existing cardiac disease. Of patients diagnosed with PE in PIOPED, >90% had dyspnea, tachycardia, or pleuritic chest pain. Similarly, although chest radiographic findings alone are not sensitive or specific for PE, they are essential for diagnosing conditions that can mimic PE clinically. The most common radiographic findings in patients with PE were atelectasis or parenchymal opacity. Furthermore, chest radiographic findings heavily influence the criteria utilized for estimating the probability of PE based on lung scan patterns. For example, in patients with COPD, the sensitivity of a high probability V/Q scan is significantly lower than in patients with no cardiopulmonary disease. The more severe the underlying cardiopulmonary disease, the higher the likelihood of the scan will be of intermediate probability. Intermediate probability occurred in 60% of patients with COPD in PIOPED, compared with only 13% of patients with a normal chest x-ray.27
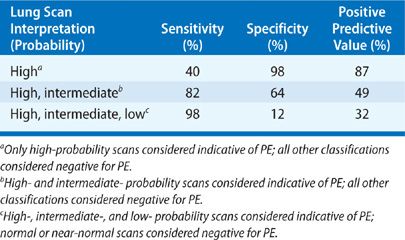
The sensitivity, specificity, and PPV from PIOPED of V/Q lung scans in detecting acute PE are presented in Table 32-1.
TABLE 32-1 Sensitivity, Specificity, and Positive Predictive Value of Lung Scans in Detecting Pulmonary Embolism in Patients Enrolled in PIOPED
All patients enrolled in PIOPED were to undergo a V/Q scan and pulmonary arteriogram. However, a large number of patients with low probability or normal V/Q scans did not undergo pulmonary arteriography to avoid additional risks. Therefore, there was a selection bias toward patients with intermediate or high probability scans, leading to an overestimation of the sensitivity, but underestimation of the specificity of V/Q scanning. In an analysis of the angiography results in PIOPED, PE occurred more frequently on the right than on the left, and more commonly in the lower lung zones. One of the limitations of V/Q scanning in PIOPED was the interobserver variability in scan interpretation. As expected, agreement among readers was excellent for normal, high, and very low probability V/Q scans (92%–95%). However, it was not as good for intermediate and low probability studies.27
Use of anatomic lung segment reference charts has been shown to reduce interobserver disagreement when interpreting scans. Other interpretative pitfalls include false-negative and -positive readings. False-negative interpretations (i.e., low probability read with PE present) do occur, and patients who have a recent history of immobilization (bed rest for 3 days), recent surgery, trauma to the lower extremities, or central venous instrumentation are particularly at risk. In patients with low or very low probability scans who have none of the aforementioned risk factors, the prevalence of PE is only 4.5%. Conversely, in patients with low or very low probability scans and one or more of the risk factors, the prevalence of PE is 12% and 21%, respectively (Table 32-2).
TABLE 32-2 Risk Factors and Prevalence of Pulmonary Embolism in Patients with Low Probability and Very Low Probability Lung Scans Enrolled in PIOPED
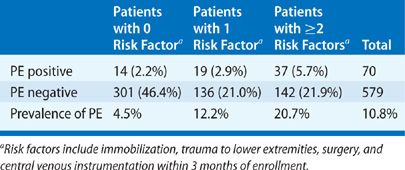
Patients with false-negative lung scans tend to have nonocclusive subsegmental thrombi and a low pulmonary clot burden. The prognostic value of a low probability scan is excellent, particularly in patients with a low clinical pretest likelihood of disease or negative lower leg ultrasound.28 The most common cause of V/Q mismatch in patients who do not have acute PE is chronic or unresolved PE. Other causes include compression of the pulmonary vasculature (e.g., from mass lesions, lymphadenopathy, or mediastinal fibrosis), vessel wall abnormalities (e.g., pulmonary artery tumors or vasculitis), nonthromboembolic intraluminal obstruction (e.g., tumor emboli or foreign body emboli), and congenital vascular abnormalities (e.g., pulmonary artery agenesis or hypoplasia).
In patients who have unilateral V/Q mismatch (hypoperfusion or absent perfusion) within an entire lung or in multiple contiguous segments and normal perfusion in the contralateral lung, extrinsic compression of the pulmonary vasculature, congenital abnormalities, or proximal PE should be considered. Patients with a suspected false-positive scan or unilateral V/Q mismatch often require further imaging using CTA, even though emboli are frequently multiple (in 90%) and bilateral (in 85% of cases), and unilateral decreased or absent perfusion to one lung is uncommonly the result of PE.
Shortly after the PIOPED study, helical CTA was introduced.29 This led to PIOPED II, which is the largest multicentered, prospective, outcome-based study to date designed to assess the accuracy of CTAin evaluation of acute PE in comparison to the composite reference standard.30
In PIOPED II, CTA had a sensitivity, specificity, PPV, and NPV of 83%, 96%, 86%, and 95%, respectively, in detecting acute PE.30 Study results suggested that the predictive value of CTA is highly concordant with the pretest clinical probability of PE using Wells’ criteria.25 The PPV of PE with a positive CTA was 97% for a main or lobar artery, 68% for a segmental vessel, and 25% for a subsegmental branch. The probability of a false-negative chest CTA is minimal in large PE, but false-negative findings may occur in the small group of patients with clot limited to subsegmental arteries. The PPV of PE in patients with positive CTA and high, intermediate, or low clinical probability was 96%, 92%, 58%, respectively. NPV of PE in patients with negative CTA and low, intermediate, or high clinical probability was 96%, 89%, and 60%, respectively.
The limitations of the study included use of 4- to 16-slice multidetector CT (mostly 4-detector) and use of noninvasive diagnostic test in the reference standard. In contrast to original PIOPED population, of whom 68% were inpatients, PIOPED II included only 11% inpatients. Inpatients are more likely to have abnormalities on chest radiographs, which would potentially interfere with optimal V/Q scan reading.31 Also, the accuracy of CTA would be lower if patients with inconclusive interpretations of CT (51 patients or 6%) were included, giving an overall sensitivity and specificity of 78% and 90%, respectively. The overall PPV of 86% and NPV of 95% are comparable to V/Q performance parameters. The combination of CT venography and CTA increased the sensitivity from 83% to 90% when using CTA alone. CT venography, however, showed similar results in diagnosing or excluding DVT as compression ultrasound.32
Finally, PIOPED III, a multicenter collaborative investigative trial was designed to determine the diagnostic accuracy of gadolinium-enhanced magnetic resonance angiography (Gd-MRA) of the pulmonary arteries in combination with magnetic resonance venography (MRV) of the veins of the thighs in patients with clinically suspected acute PE. In PIOPED III, most centers had difficulty in obtaining MR pulmonary angiograms of adequate quality; indeed, studies were deemed inadequate in 25% of patients.33 Adequacy of the studies varied between 11% and 52% among centers. Adequate quality images were obtained in assessing the main or lobar pulmonary arteries in 91% of patients, segmental pulmonary arteries in 87%, and subsegmental branches in 73%. A technically adequate MRA had a sensitivity of 78% and specificity of 99%. These findings led the PIOPED III investigators to conclude that MRA should only be considered at centers that routinely perform it well, and for patients who have contraindications to other standard tests.
 V/Q SCAN INTERPRETATION AND MODIFICATIONS TO ORIGINAL PIOPED CRITERIA
V/Q SCAN INTERPRETATION AND MODIFICATIONS TO ORIGINAL PIOPED CRITERIA
Several diagnostic schemes have been suggested for interpretation of V/Q scans. The original PIOPED criteria were developed to interpret scans generated from the study based upon experience gathered over the preceding decade. A PE is characterized by V/Q mismatch, which is an area of normal ventilation corresponding to a segmental, wedge-shaped area of decreased or absent perfusion extending to the pleural surface. The segmental defect should correspond to vascular anatomy. Due to the high proportion (44%) of intermediate probability V/Q scans in PIOPED34 and interobserver disagreements, several revisions and modifications have been made to the original criteria.
One nuance in V/Q scan interpretation is the so-called “gestalt interpretation,” which is based on an experienced nuclear medicine physician’s subjective estimate of the likelihood of PE (without using specific interpretation criteria). This interpretation takes into account various published lung image interpretation algorithms, clinical data, ancillary findings, and pathophysiologic features of PE, which are integrated with the individual case presentation. The gestalt interpretation has been shown to correlate well with the fraction of patients with angiographic evidence of PE in the PIOPED study, with good-to-excellent intra- and interobserver variability.35,36 Thus, experienced readers (such as the PIOPED investigators) can provide an accurate estimate of the probability of PE based on clinical, radiographic, and scintigraphic findings.
Another nuance is the “triple match,” which is a reference to matching perfusion, ventilation, and chest radiographic abnormality. Based on the PIOPED data, in the presence of a triple match, the prevalence of PE varies depending on the location of the abnormality. A triple match in the upper (prevalence of PE, 11%) or middle lung (prevalence of PE, 12%) zones is considered low probability for PE, but a triple match in the lower lung zones should be interpreted as intermediate probability (prevalence of PE, 33%).37
Yet another nuance is the “stripe sign,” which describes a rim of perfused lung tissue between the perfusion defect and adjacent pleural surface.38 In PIOPED, this sign excluded the diagnosis of PE within the affected zone in 93% of cases. Hence, in the absence of other perfusion abnormalities, a V/Q scan with a stripe sign should be considered very low probability for PE. A single, moderate-sized V/Q mismatch was found to harbor PE in 36% of cases in PIOPED39; if present, the V/Q scan should be described as intermediate probability for PE. A single matched V/Q defect (of any size) was found to correspond to PE in 26% of cases in PIOPED; such a finding should be classified as intermediate probability.39
The limitations in original PIOPED led the investigators to revise the original interpretation criteria for easier application and better integration of clinical pretest probability of PE. Modifications to the original PIOPED interpretation criteria were used in the PIOPED II trial (Table 32-3),40 which decreased the number of intermediate scan readings (73.5% of patients had V/Q scans with definitive interpretation) and provided a more accurate assessment of angiographically proven PE than the original criteria (sensitivity increased to 83%; specificity essentially unchanged at 96%).
aCriteria generated after completion of prospective study.
On the other hand, the PISA-PED26 criteria (Table 32-4) for interpretation of perfusion lung scans were based on diagnosing PE when there was one or more wedge-shaped perfusion defects. PE was considered absent when there was normal or near-normal perfusion or a nonsegmental, non–wedge-shaped perfusion defect.41 All other scans were called nondiagnostic.
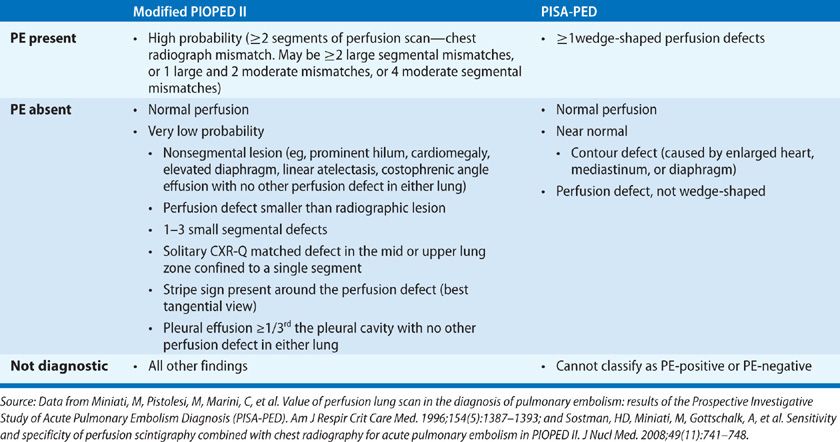
According to modified PIOPED II criteria (Table 32-4) established in 2008, PE was diagnosed when there were equivalent of two large, segmental perfusion scan–chest radiograph mismatches (which may include one large and two moderate segmental mismatches, or four moderate segmental mismatches).41 PE was considered absent when there was normal perfusion. PE was deemed very low probability when there was a nonsegmental perfusion defect smaller than the corresponding radiographic lesion, one to three small segmental defects, a solitary matched chest x-ray abnormality and perfusion defect in the mid or upper lung zones, a stripe sign, or a pleural effusion ≥1/3 of the pleural cavity with no other perfusion defect in either lung. The scan was considered nondiagnostic with all other findings.
When perfusion scans obtained in PIOPED II were reinterpreted using a composite reference standard (all PIOPED II patients were eligible for this study if they had a diagnosis based on CTA or digital subtraction angiography [DSA], an interpretable perfusion scan and chest radiograph, and a positive Wells’ score), investigators found only 21% of patients with nondiagnostic results using revised PIOPED II criteria.41 Only 11% patients had nondiagnostic perfusion scans among the 72% of patients with normal or near-normal chest radiographs. The prevalence of PE in the sample was 19%. Using the modified PIOPED II criteria, and after excluding nondiagnostic perfusion scans, the sensitivity of “PE present” reached 85%, and the specificity of “PE absent” reached 93%. When using PISA-PED criteria, none had nondiagnostic perfusion scans; the sensitivity of a “PE present” scan was 80% and the specificity of “PE absent” scan was 97%. Based on these findings, it was postulated that perfusion scintigraphy combined with chest radiography can provide diagnostic accuracy similar to CTA at a lower cost and, most importantly, with a lower radiation dose.41
 SPECT V/Q IMAGING FOR THE EVALUATION OF PULMONARY EMBOLISM
SPECT V/Q IMAGING FOR THE EVALUATION OF PULMONARY EMBOLISM
Single-photon emission tomography (SPECT) V/Q imaging has several advantages over planar imaging, including higher contrast resolution and avoidance of overlapping small perfusion defects by normal tissue, particularly at the lung bases.42 In the last two decades, technologic advances have been made with SPECT and new radiopharmaceuticals developed for SPECT ventilation studies, such as 99mTc Technegas.43 Due to the availability of Technegas outside the United States, most SPECT V/Q scanning is performed in Australia and Europe (Figs. 32-3 and 32-4).
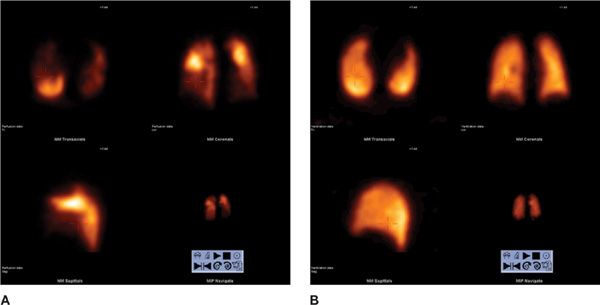
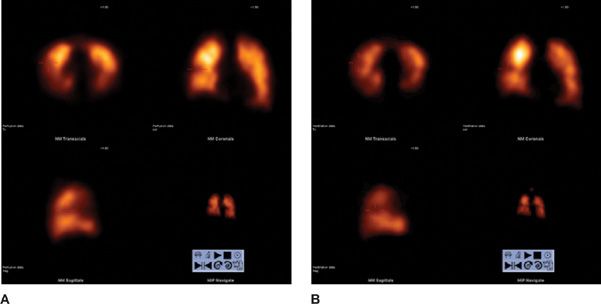
Figure 32-3 High probability V/Q SPECT scan for pulmonary embolism. Perfusion scan using 99mTc MAA in transaxial, coronal, and sagittal views (A) shows large segmental defects in both lungs. Ventilation scan using 99mTc Technegas® aerosol in transaxial, coronal, and sagittal views (B) is within normal limits. This combination of findings (mismatch) is consistent with pulmonary embolism.
Figure 32-4 V/Q SPECT scan for pulmonary embolism. Perfusion scan using 99mTc MAA in transaxial, coronal, and sagittal views (A) and ventilation scan using 99mTc Technegas® aerosol in transaxial, coronal, and sagittal views (B) show matched defects.
Total acquisition time for the SPECT V/Q examination is between 20 and 30 minutes for a dual-headed camera.44 The advantages of SPECT over planar imaging in the evaluation for PE have been reported in several studies.45–48 Importantly, SPECT V/Q scans have been reported by most investigators to be ≤3% nondiagnostic.44 When compared to planar V/Q scans, SPECT V/Q scans show more and better delineated mismatched defects, better quantification, and less interobserver variation.46
In most studies, sensitivity (80%–100%) of SPECT V/Q scanning is higher than planar V/Q scan, but the specificity (93%–100%) is not always higher.44 Some investigators have also suggested the use of combined SPECT V/Q scan and low-dose CT scanning to improve accuracy in diagnosing PE.49 The interpretation criteria, however, for SPECT V/Q scanning are not yet clearly defined or universal (i.e., it remains unclear whether modified PIOPED or PISA-PED criteria can be simply transposed from planar to SPECT V/Q scanning). Despite the many advantages of SPECT, substitution of planar V/Q by SPECT V/Q scan in patients with suspected PE remains controversial.31,50,51
There is a growing interest in “positive” imaging based on the use of antibody fragments and radiolabeled peptides directed against components of thromboemboli and glycoprotein IIb/IIIa receptors on the surface of activated platelets. 99mTc-labeled apcitide is a synthetic peptide that binds with high affinity and specificity to the glycoprotein IIb/IIIa receptor on the membrane of activated platelets.52 The main advantage of the agent is its ability to distinguish between acute and chronic DVT.
Several 99mTc-labeled peptides directed against activated platelets are currently under investigation in the evaluation of patients with suspected PE. Radiolabeled peptide imaging has the potential to serve as a single, comprehensive modality in the evaluation of patients with VTE.
Finally, another agent under investigation is 99mTc-labeled anti–D-dimer (DI-80 B3) monoclonal antibody Fab′ fragment that binds specifically to thromboemboli. In a recent prospective, multicenter study to investigate the sensitivity and specificity of 99mTc DI-80 B3/SPECT in patients with suspected acute PE, 99mTc-DI-80 B3/SPECT had a sensitivity of 76.2% and a specificity of 90.5%.53 At the current time, further studies and development of newer radiopharmaceuticals are required to fully realize this potential.
 RECOMMENDATIONS FOR THE USE OF V/Q SCANNING AND CT ANGIOGRAPHY IN SUSPECTED PULMONARY EMBOLISM
RECOMMENDATIONS FOR THE USE OF V/Q SCANNING AND CT ANGIOGRAPHY IN SUSPECTED PULMONARY EMBOLISM
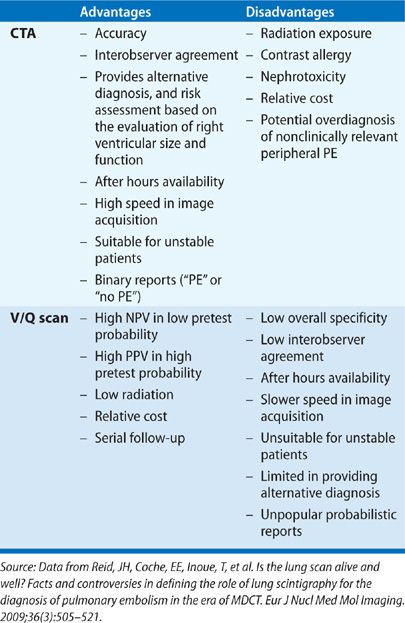
Over the past decade, significant technical improvements have occurred in both CTA and V/Q scanning. However, use of CTA of the chest in patients with suspected PE has increased significantly and has markedly surpassed use of V/Q scanning.54 Although studies show equivalent outcome-based results, the ready availability of CTA, particularly after hours, and the bias toward anatomic, rather than functional imaging, has made CTA the favored diagnostic test for PE.55
In a recent large prospective randomized study with high pretest probability and/or positive D-dimer levels, the false-negative rates for V/Q scan and CTA were very close at 1% and 0.4%, respectively.56 Despite the fact that CTA can detect more small emboli than V/Q scan,56 the risk of recurrent PE and death have not decreased during the CTA era.57 In fact the ability of sophisticated new multidetector CT scans to detect emboli in subsegmental branches of the pulmonary arteries has created a healthcare challenge, pushing clinicians to now treat patients who have incidental, asymptomatic pulmonary emboli, whose natural history and optimal management are currently unknown (except in cancer patients in whom these emboli carry a poor prognosis).58,59 These scans are usually ordered in the emergency department with no particular pretest diagnosis. The same logic will probably be true with SPECT V/Q scan, as it is not yet clear whether treating small, peripheral PE will confer a benefit unless the patients have significant clot burden and limited cardiovascular reserve.34
In patients with a normal chest radiograph, the V/Q lung scan is an effective, noninvasive initial study. However, in patients with significant chest radiographic abnormalities, CTA is more likely to provide a definitive diagnosis of PE or an alternative diagnosis, as well as a risk assessment of PE based on the evaluation of right ventricular size and function.60,61 Furthermore, the combination of CTA and CT venography has the potential to provide a single, comprehensive evaluation of patients with suspected VTE, albeit with increased radiation exposure. V/Q scan remains an important alternative to CT in patients with contrast allergy or renal failure.
V/Q scan may be the modality of choice to evaluate patients with chronic thromboembolic disease and in providing follow-up of PE after therapy.31 There is concern regarding the high radiation exposure, particularly to the female breast, associated with chest CTA.62,63 CTA delivers a minimum radiation dose of 20 mGy (2.0 rad) to the breasts of an average-sized woman,64 whereas breast irradiation with V/Q scan is approximately 0.28 to 0.9 mGy.65 Furthermore, concern exists that increasing use of CTA may result in an increased incidence of radiation-related cancer in the future.62 The potential latent carcinogenic effects of such radiation exposure at this time remain unknown.
As part of the American Board of Internal Medicine’s Choosing Wisely campaign, which focused on potentially unnecessary or harmful medical tests and procedures, the Society of Nuclear Medicine recommended avoiding CTA to diagnose PE in young women with a normal chest radiograph, and consideration of a V/Q study instead.66 Considerable debate exists regarding fetal radiation doses from pulmonary CTA versus V/Q scanning.67 During pregnancy, when only a perfusion scan with 50 MBq of 99mTc MAA is used, the fetal absorbed dose is 0.1 to 0.2 mGy. It is estimated that a 16-slice MDCT scan gives an absorbed fetal dose of 0.24 to 0.66 mGy during the first trimester.68 A comparison between CTA and V/Q scan for the evaluation of PE is summarized in Table 32-5.
In summary, based upon results from prospective and outcome-based studies, the following conclusions can be drawn regarding the use of V/Q scan and CTA in evaluating patients with suspected PE:
1. Proper pretest clinical probability scoring is important irrespective of the modality used. When imaging results for either V/Q scan or CTA are discordant with the pretest probability, the alternative test should be recommended.
2. A normal V/Q scan excludes the diagnosis of clinically significant PE.
3. Patients with very low or low probability scans and a low clinical likelihood of PE have a low (<5%) prevalence of PE and generally do not require pulmonary angiography or anticoagulation.
4. Patients with very low or low probability scans, intermediate or high clinical likelihood of PE, and negative serial noninvasive venous studies of the lower extremities generally do not require anticoagulation. In selected cases, CTA is helpful in excluding PE and providing an alternative diagnosis.
5. Clinically stable patients with intermediate probability scans require noninvasive venous studies of the legs; if negative, CTA is required for definite diagnosis of PE.
6. A clinically stable patient with a high probability scan and high clinical likelihood of PE, or a patient suspected of having a false-positive scan, requires treatment; no further diagnostic tests are required to confirm the diagnosis.
7. Clinically stable patients with high probability scans and a low clinical likelihood of PE require noninvasive venous studies of the legs; if negative, CTA may be required for definitive diagnosis.
8. V/Q scintigraphy has a high NPV and should be used particularly where low radiation dose is desirable, such as in young female with normal chest radiograph, in an outpatient with low clinical probability plus normal chest radiograph, a patient with high clinical probability plus normal chest radiograph, a patient with prior contrast anaphylaxis and strong allergic history, and a patient with renal failure or multiple myeloma. Since, in most patients, PE can be excluded on the basis of a normal perfusion pattern, to minimize radiation to the fetus in a pregnant patient, a 1- to 2-day protocol is suggested. Perfusion-only scans should be performed on day 1, using a reduced dose of 99mTc MAA.
9. V/Q scan is preferred over pulmonary CTA for follow-up of PE and investigation of the etiology of pulmonary hypertension.
10. If after-hours imaging is not available and a patient has a high clinical suspicion of PE, a reasonable approach includes administering a single dose of low–molecular-weight heparin and imaging the patient the next morning. This strategy may be particularly advisable for performing V/Q scintigraphy in young women to avoid the excessive breast radiation exposure associated with CTA.
11. If there is a contraindication to V/Q scan or CTA, MRA of the pulmonary artery may be performed in centers that perform it well. There is always concern about nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy, which occurs rarely in patients with poor renal function who receive gadolinium-containing contrast material.69,70
12. Whenever possible V/Q scintigraphy should be interpreted as either “positive for PE,” “nondiagnostic,” or “no evidence of PE.”
EVALUATION OF CHRONIC THROMBOEMBOLIC PULMONARY HYPERTENSION
Chronic thromboembolic pulmonary hypertension (CTEPH) is defined as the persistence of pulmonary hypertension (defined as a mean pulmonary artery pressure >25 mm Hg) after a single or recurrent PE (see Chapter 73). CTEPH is a serious, underdiagnosed disease and the only cause of pulmonary hypertension that can be potentially cured by pulmonary endarterectomy (PEA). Estimates are that between 0.5% and 3.8% of patients eventually develop CTEPH after an acute PE; CTEPH may develop in up to 10% of those with a history of recurrent PE.71,72
It is not entirely clear why some patients with a history of acute PE go on to develop CTEPH. The risk of developing CTEPH increases in patients with a history of prior PE, presentation at a younger age, larger perfusion defects, and idiopathic PE at presentation.71 Diagnosis is the key to survival in CTEPH, as without appropriate treatment long-term prognosis is poor. There is positive correlation between increased mean pulmonary artery pressure and mortality.73 Unfortunately, the clinical features, laboratory studies, chest radiograph, electrocardiogram, and echocardiogram are often unreliable in distinguishing CTEPH from primary and nonthromboembolic secondary pulmonary hypertension.
In most centers, conventional pulmonary angiography remains the gold standard imaging study to confirm the diagnosis of CTEPH and determine whether surgical intervention is indicated; chest CTA and MRA provide complementary information, if needed.
V/Q lung scanning is a safe, noninvasive technique that facilitates selection of patients with pulmonary hypertension for pulmonary angiography to confirm the diagnosis of chronic PE. V/Q scanning remains one of the most important diagnostic tests to help distinguish CTEPH from other forms of pulmonary hypertension. In a retrospective analysis, V/Q scanning had a sensitivity of 97.4% for detection of chronic thromboembolic disease, while that for CTA was only 51%.74
Patients with CTEPH usually have at least one, and often several, segmental or larger, mismatched perfusion defects.75 To prevent potential adverse hemodynamic effects when performing V/Q scans in patients with pulmonary hypertension, the number of 99mTc MAA particles administered should be reduced. V/Q scanning may underestimate the magnitude of central vascular occlusion by chronic emboli, as determined at conventional pulmonary angiography or thromboendarterectomy.76
Most patients with primary or secondary nonthromboembolic pulmonary hypertension have low probability scans. The distribution of 99mTc MAA particles within the lungs is diffuse and nonhomogenous. Patients with CTEPH rarely, if ever, have normal or very low probability scans. Thus, a low probability V/Q scan effectively excludes chronic thromboembolism as the cause of pulmonary hypertension. In patients with primary pulmonary hypertension, areas of reverse mismatch on the V/Q scan have been shown to correlate with areas of mosaic-increased attenuation on high-resolution CT scans.77 In a small study of 55 patients suspected of having CTEPH, the detection rates for central emboli with CTA were similar to conventional pulmonary angiography, although detection of segmental disease was superior with conventional pulmonary angiography.78
Even though SPECT is more sensitive than planar perfusion lung scanning for identifying obstructed segments in CTEPH, the technique still underrepresents the true extent of the vascular occlusions in CTEPH.79 Although CTA and MRA may provide complementary information, they often miss the eccentric lesions of CTEPH. Accordingly, the V/Q scan remains the screening study of choice for CTEPH. Any patient with unexplained pulmonary hypertension should be evaluated for the presence of CTEPH; a V/Q scan is recommended as screening method of choice.80
QUANTITATIVE VENTILATION–PERFUSION LUNG SCANNING
In patients undergoing pulmonary resection, bronchoscopic lung volume reduction, or lung transplantation, quantitative V/Q lung scanning is a useful method for determining regional lung function and, thus, predicting postoperative pulmonary function (see Chapter 103).81–83
Currently, the presurgical assessment to predict response to lung volume reduction surgery (LVRS) in patients with emphysema is mainly performed using chest CT scanning,84 which provides an anatomic diagnosis of emphysema, delineates its extent and distribution, and detects any other incidental findings that may represent a contraindication to the procedure. A V/Q scan can provide additional information concerning the heterogeneity and distribution of the functional disruption caused by emphysema.84 Patients with advanced, upper lobe–predominant emphysema derive the most benefit from LVRS,85 and perfusion scintigraphy is a commonly available test for assessing the distribution of emphysema by reflecting regional lung function.86 Rectangular regions of interest over the anterior and posterior scintigraphic images of each lung are divided into upper, middle, and lower zones of equal craniocaudal height, and the geometric mean for a given zone is obtained and presented as the percent perfusion to that zone.87
In patients being considered for lung cancer surgical resection, a V/Q scan can predict postoperative pulmonary function and enable assessment of the risk of surgery for patients with borderline pulmonary function.88 The predicted postoperative forced expiratory volume in 1 second (ppo FEV1) is calculated by multiplying the preoperative value by the ratio of the counts in the remaining lung to total lung activity (Fig. 32-5).89,90
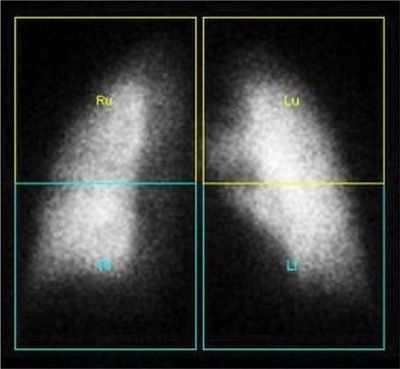
Figure 32-5 Quantitative perfusion lung scan. Regional perfusion can be quantified by outlining regular or irregular regions of interest and generating ratios that correspond to percent of total pulmonary function. Images shown were analyzed by dividing each lung into two equal rectangles.
Lung perfusion scintigraphy can be used to quantify the degree of right-to-left shunting due to patent foramen ovale, atrial septal defect, elevated right heart pressures, or hepatopulmonary syndrome (HPS).91,92 The number of injected 99mTc MAA particles should be reduced in patients with suspected right-to-left shunt. Normally, <5% of 99mTc MAA is taken up in the brain, but in HPS, fewer MAA particles are entrapped in pulmonary vasculature and are able to enter the systemic circulation and lodge in different organs (thyroid, spleen, liver, kidneys, and brain), leading to >6% uptake in the brain (Fig. 32-6). The major disadvantage of perfusion scintigraphy is that it cannot distinguish intracardiac from intrapulmonary right-to-left shunt. Estimation of the right-to-left shunt fraction is calculated using the following equation: 100 × (TBC – TLC)/(TBC), where TBC is total body count and TLC is total lung count. A less precise alternative method of right-to-left shunt quantification derives an estimate of total body counts from the measurement of renal and/or cerebral activity.
Figure 32-6 Right-to-left shunt in a patient with hepatopulmonary syndrome. Perfusion scan using 99mTc MAA is showing particles in the systemic circulation lodged in the kidneys.
ASSESSMENT OF ALVEOLAR–CAPILLARY MEMBRANE PERMEABILITY AND MUCOCILIARY CLEARANCE
The clearance rate of inhaled 99mTc DTPA aerosol from the lungs can be measured using a gamma camera and provides an index of lung epithelial permeability.11 Aerosols of relatively small aerodynamic diameter (e.g., 99mTc DTPA) are deposited largely within the small airways and alveoli; the normal half time of 99mTc DTPA wash out from the lungs is about 80 minutes.12 In the presence of epithelial alveolar damage, the clearance of 99mTc DTPA is accelerated.11 Examples of such injuries include amiodarone toxicity, inhalation damage in fire victims, pneumoconiosis, idiopathic pulmonary fibrosis, collagen vascular diseases, sarcoidosis, acute respiratory distress syndrome, and pneumocystis pneumonia.11,93 Cigarette smoking or physiologic factors, such as posture and exercise, also influence epithelial lung clearance.11,94 Since increased alveolar–capillary membrane permeability is relatively nonspecific, 99mTc DTPA aerosol clearance studies have been utilized only to assess the effects of therapy in patients with known pulmonary diseases.
Mucociliary clearance is a primary physiologic defense mechanism, protecting the lungs from damage caused by inhaled particles and microorganisms. Determination of mucociliary clearance may be obtained after the inhalation of relatively large aerosolized particles, followed by measurement of the rate of clearance using a gamma camera. The rate of mucociliary clearance depends on several factors, including ciliary activity and mucus production. Inhaled particles, such as 99mTc MAA or 99mTc sulfur colloid, tend to be deposited within the proximal airways.95 The normal mucociliary clearance half time is approximately 24 hours. Delayed mucociliary clearance is seen in patients with airway inflammation (e.g., cystic fibrosis, COPD, asthma, or viral respiratory tract infections), following bronchial surgery, or after irradiation.
Scintigraphic assessment of alveolar–capillary membrane integrity and mucociliary clearance has not gained wide clinical use,93 but it is employed in the development of orally inhaled drugs that are administered by inhalation by assessing their deposition patterns, extent of delivery, and depth of penetration into the lungs.96 In addition, the technique is applicable in quantification of the impact of new drugs on the rate of mucociliary clearance—a key biomarker for products in development for the treatment of respiratory diseases.97
POSITRON EMISSION TOMOGRAPHY
PET is used in assessing nonmalignant thoracic disease, as well as pulmonary nodules and known or suspected malignancy. Following are considered basic principles and the role of PET in evaluating inflammatory lung diseases in the overall population, and infections in immunocompromised hosts. Comparison is made with other diagnostic tests, including 67Ga-citrate scanning. The role of PET in assessment of lung nodules and cancer is discussed in a separate section (see Role of Positron Emission Tomography in the Assessment of the Solitary Pulmonary Nodule and Lung Cancer).
 BASIC PRINCIPLES
BASIC PRINCIPLES
PET is a nuclear medicine imaging modality that provides a 3D image of molecular processes in the body. A PET scan detects gamma rays emitted by positron-emitting tracers. The most commonly used radiopharmaceutical in clinical PET is 2-[18F] fluorodeoxy-D-glucose (FDG), a fluorine-labeled glucose analog with a favorable, 110-minute half-life. FDG competes with glucose for transport into cells and for enzymatic phosphorylation by hexokinase. Unlike glucose, once phosphorylated to FDG-6-phosphate, FDG does not undergo further metabolism; rather, it is trapped inside the cell, and its net accumulation allows for detection by PET.98
FDG uptake is proportional to the metabolic activity of the cells that have undergone malignant transformation and, as such, have increased glucose transport and metabolism and increased hexokinase activity. FDG accumulation also depends on various factors, including cellular mitotic rates, level of hypoxia (hypoxia-inducible factor-1-alpha upregulates glucose transport receptors), and degree of cell differentiation (well-differentiated tumors have low FDG uptake).99 In addition, inflammatory cells share many of the same features as malignant cells; therefore, FDG-PET imaging can be employed to assess infectious and inflammatory processes and diseases. FDG-PET imaging is performed in the fasting state to minimize competitive inhibition of FDG uptake by circulating glucose in the plasma (hyperglycemia may result in decreased FDG accumulation in either malignant or inflammatory cells).
A key advantage of PET over conventional imaging techniques is the possibility of accurate quantification of the ongoing metabolic activities in normal or diseased states. The most common means used for this purpose is measurement of the standardized uptake value (SUV), a semiquantitative expression of the intensity of FDG accumulation in a region of interest (ROI) assigned on the PET scan. SUV normalizes the amount of FDG accumulation in an ROI to the total injected dose and the patient’s body weight. It is calculated by dividing the mean activity within a selected region (or volume) of interest (in mCi/mL) by the injected dose (in mCi/kg). SUVmax is derived from the single voxel showing the highest uptake within a defined ROI, which typically represents the most metabolically active part of a tumor or inflammatory process.100,101 However, it is important to note that several factors can impact SUV, including extravasation of the compound administered, hyperglycemia, respiratory motion, time from injection, and size of ROI.
Today, the mainstay for PET imaging is integrated PET/CT scanners, which allow combining metabolic and structural imaging modalities into a single device. Based on existing data from the past decade, PET/CT scans have been shown to be superior to images generated from separate PET and CT studies for assessing a multitude of disorders.102 The newest generation of PET/CT scanners contain a state-of-the-art multidetector CT machine that provides up to 128 slices, and time-of-flight PET technologies that reconstruct high-quality images using low FDG doses and short scan times, especially in large patients.103 PET/CT has some limitations related to attenuation artifacts, leading to false-positive results on corrected images. In addition, motion and misregistration between PET and CT images can also result in major artifacts in regions adjacent to the heart and diaphragm.104 Combined PET/MRI systems are now commercially available, but their added potential clinical benefit has not yet been validated.105 This is particularly true in thoracic disorders.
 ROLE OF POSITRON EMISSION TOMOGRAPHY IN PULMONARY INFLAMMATION AND INFECTION
ROLE OF POSITRON EMISSION TOMOGRAPHY IN PULMONARY INFLAMMATION AND INFECTION
FDG-PET has been used in the evaluation of patients with a variety of inflammatory disorders, including venous thromboembolic disease, infections in immunocompromised hosts, and other noninfectious inflammatory conditions. Each of these entities is discussed in subsequent sections.
Potential Role for FDG-PET in Evaluating Suspected Venous Thromboembolism
In recent years, FDG-PET/CT has been explored in many inflammatory conditions. Activated inflammatory cells demonstrate increased FDG uptake due to increased numbers of cell surface glucose transporters following cellular stimulation by various cytokines.106 Similar biochemical changes are also seen with VTE.
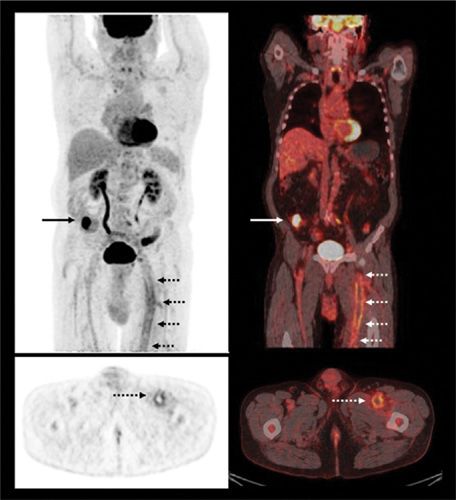
Based on our own experience and that reported in the literature,107 the potential value of PET in assessing VTE is clear for several reasons. Current imaging techniques, which are based on structural detection of thrombi, are limited; DVT and PE are only two manifestations of VTE, which may occur in parts of the venous system (e.g., within the pelvis) that are not easily accessible by conventional methods. This limitation also applies to the detection of occult disease (e.g., cancer), which is often a key factor in developing VTE (Fig. 32-7). Furthermore, structural imaging techniques cannot differentiate among different phases of thrombus formation, which may hold therapeutic implications.
Figure 32-7 Left femoral thrombosis on FDG-PET/CT scan. Maximum intensity projection PET scan (top left), transaxial PET scan (bottom left), fused coronal PET/CT scan (top right), and fused transaxial PET/CT scan (bottom right) in a patient with venous thromboembolism having a FDG-PET/CT in search of underlying malignancy. Increased FDG uptake is seen along the acutely thrombosed vein (interrupted arrows), and focally increased FDG uptake is seen in the cecum (solid arrows) corresponding to a biopsy-proven colon cancer.
We believe that many of these shortcomings will be overcome by using FDG-PET/CT. Several case reports have addressed the incidental detection of VTE with FDG-PET/CT in patients with cancer or bacteremia.108,109 Inflammatory processes also play a key role in nonmalignant, noninfectious VTE, that is, local vessel wall inflammation and inflammatory components, including activated leukocytes in the thrombus itself.
In a small, prospective, proof-of-concept series,110 both DVT and PE were shown to be visible on FDG-PET/CT. All patients with proved acute lower extremity DVT had marked FDG uptake along the venous wall at the site of thrombus. Diagnosis was established by positive compression ultrasound in patients with symptoms less than a week; no history of cancer, infection, or prior DVT; and a high clinical probability by Wells’ DVT score. Conversely, none of the control patients with suspected, but disproved, DVT had FDG uptake in any part of the venous vasculature. The diagnosis was disproved by negative compression ultrasound in patients with symptoms less than a week; no history of cancer, infection, or prior DVT; and a low clinical probability by Wells’ DVT score. Results were more equivocal in PE with some patients with high probability V/Q scans showing no FDG uptake in the pulmonary arteries. However, this may be attributed to significantly longer symptom duration (i.e., months) in some patients. It may, in fact, be that FDG-PET/CT was truly negative for active thrombi, since recent data suggest a significant decrease in FDG avidity over time.
In a pivotal study of 12 patients with confirmed proximal thrombosis in the lower extremity, the authors not only found a specificity of 87.5% to 100% and sensitivity of 87.5% to 100% (depending on SUVmax threshold used), but also a steady decrease in SUVmax and possible complete normalization within 3 months.111 These findings are in accordance with VTE pathophysiology: The thrombus may be classified as acute when inflammatory cells and mediators are abundant, subacute with subsiding inflammation and increasing fibrosis, and finally, chronic with recanalization and loss of inflammation. This pathophysiology will have significant clinical implications, since active thrombi need therapy, while unresolved old clots require no treatment. However, a substantial fraction of patients with VTE experience symptoms of recurrence, and in as many as half the morphologic features suggestive of the diagnosis remain for years after the first event.107 FDG-PET/CT likely will differentiate new, active clots from old, inactive ones and facilitate an individualized therapeutic approach.
Although the literature is still sparse, the role of FDG-PET/CT imaging appears to be very promising in VTE and may contribute significantly by detecting clots at any location throughout the body, by early diagnosis of underlying malignancy, and by differentiating acute from chronic thrombi.
Imaging of the Thorax in the Immunocompromised Host
In the last three decades, 67Ga-citrate and white blood cells labeled with 111In or 99mTc were considered as appropriate agents for imaging pulmonary infection and inflammation. However, due to poor image quality, laborious labeling techniques, and the requirement for imaging several days after injection, imaging with either 67Ga-citrate or radiolabeled white blood cells is now considered obsolete. In recent years these methods have been largely replaced by FDG-PET/CT in the evaluation of suspected infection or inflammation in many anatomic locations. This is also the case in the lungs, where FDG-PET/CT is employed for evaluating suspected pulmonary infections, fever of unknown origin in the immunocompromised host (Fig. 32-8), opportunistic infections in patients infected with HIV (Fig. 32-9), and a multitude of inflammatory disorders.112 In spite of limited data, an early pilot study also suggested that FDG-PET might be helpful in determining treatment response in patients with tuberculosis.113
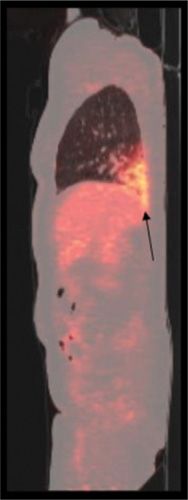
Figure 32-8 Fever of unknown origin in an immunocompromised patient with a kidney transplant. Conventional chest x-ray was normal. Fused sagittal FDG-PET/CT image shows characteristically increased FDG uptake in the right lower lung (arrow) consistent with lobar pneumonia.
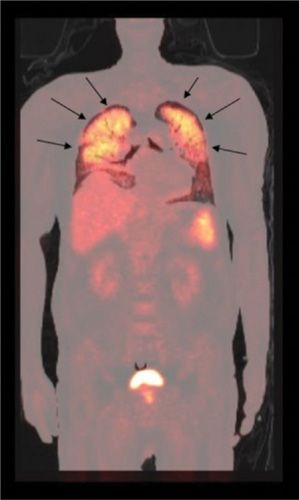
Figure 32-9 Pneumocystis jirovecii pneumonia. Diffuse, markedly increased FDG lung uptake bilaterally (arrows) in a patient with human immunodeficiency virus.
Noninfectious Inflammatory Lung Disease
67Ga-citrate lung imaging has been used to quantify the degree of alveolitis in various interstitial lung diseases, particularly sarcoidosis and idiopathic pulmonary fibrosis. Unfortunately, pulmonary accumulation of 67Ga in idiopathic pulmonary fibrosis was found not to be reliable in predicting the response to treatment or prognosis.114 Also, in these settings, FDG-PET/CT appears to be substantially superior to 67Ga. A novel potential indication is the assessment of inflammatory disease activity in COPD using FDG-PET/CT. Even with sparse reports in the literature, the evidence points toward a correlation between the distribution and severity of emphysema and the degree of FDG uptake, which may have significant implications for managing patients with this serious disorder.
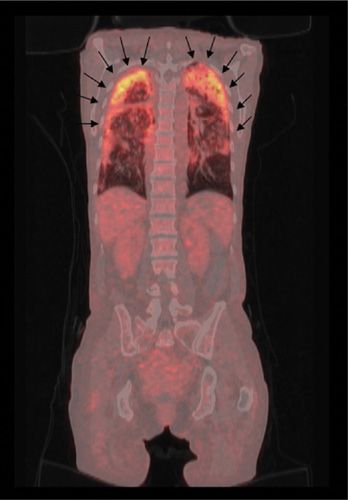
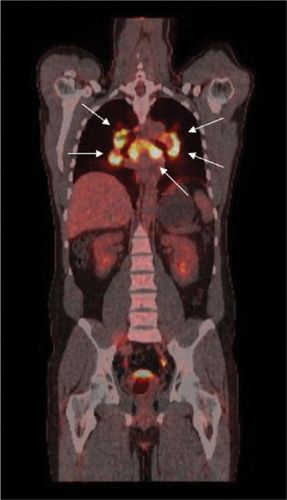
Sarcoidosis is a multisystem inflammatory granulomatous disease, which involves the lungs in 90% of patients; it may affect any organ in the body. Scintigraphy with 67Ga-citrate has been advocated for assessment of disease activity, but in recent years, this technique has been overshadowed by FDG-PET, which has several technical advantages, including lower radiation exposure, shorter time interval between injection and imaging, and higher quality images. Hilar and mediastinal lymph nodes (as well as any other soft tissues) harboring active granulomas due to sarcoidosis accumulate FDG (Fig. 32-10). Although FDG-PET cannot distinguish sarcoidosis from other diseases, such as Hodgkin or non-Hodgkin lymphomas, the technique is quite effective in assessing the extent of disease after an initial diagnosis, thereby selecting the appropriate site for a successful biopsy. FDG-PET can also provide a means for assessing response to treatment (Fig. 32-11).
Figure 32-10 Sarcoidosis. Typical presentation of sarcoidosis on FDG-PET/CT with markedly increased FDG uptake in mediastinal and hilar lymph nodes (arrows). Similar patterns are seen in patients with lymphoma.
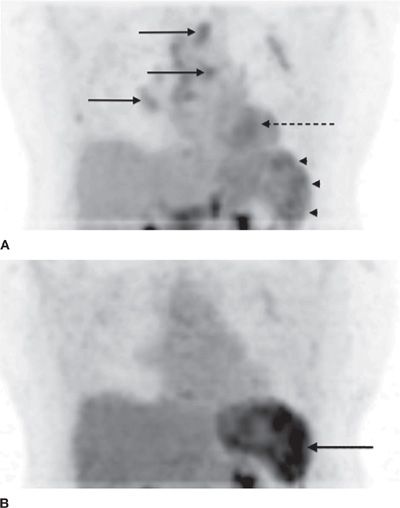
Figure 32-11 A. FDG-PET/CT images of the thorax and upper abdomen reveal significant disease activity in mediastinal and hilar lymph nodes (solid arrows), the myocardium (interrupted arrow), and the spleen (arrow heads). B. Following successful treatment, there is substantial response in the affected site in the heart and the lymph nodes. However, there is significantly increased uptake of FDG in the splenic lesions (solid arrow), which demonstrates the complexity of the biologic behavior of sarcoidosis in various organs during the course of the disease.
FDG-PET has been found to be more sensitive (97%) than 67Ga-citrate scintigraphy (88%) in detecting active sarcoidosis.115 Comparative studies have shown that FDG-PET can detect more intra- and extrathoracic lesions than 67Ga scintigraphy, with better interobserver agreement.115–118 High FDG uptake in the involved lung parenchyma correlates with disease severity as revealed by bronchoalveolar lavage.119 FDG-PET will likely have an increasing role in evaluating the efficacy of therapeutic interventions in this serious disease.118 In a recent study of 90 patients with chronic sarcoidosis and persistent symptoms, FDG-PET/CT proved helpful in detecting active inflammatory sites, especially in patients with normal ACE levels, thereby influencing adjustment of therapy.120
Pneumoconioses may be progressive, even after dust exposure has ceased. The inhaled particles activate pulmonary macrophages that secrete cytokines that mediate an inflammatory reaction, inducing fibroblast proliferation and collagen deposition. FDG is taken up by both fibroblasts and alveolar inflammatory cells. The intensity of pulmonary FDG uptake in pneumoconioses depends on whether active inflammation (increased uptake) or end-stage fibrosis (reduced uptake) predominates at the time of the scan.121 In addition, progressive massive fibrosis has been shown to be associated with increased FDG accumulation.122 The findings from FDG-PET have direct clinical implications, as therapeutic interventions are ineffective in end-stage fibrosis.
Interstitial lung disease may also be FDG-avid, but there is limited data on the use of FDG-PET in this setting (Fig. 32-12). In a small prospective study of 21 patients, FDG-PET did not allow differentiation of idiopathic from nonidiopathic pulmonary fibrosis.123 However, dual–time point FDG-PET imaging holds promise in differential diagnosis and prediction of disease progression in patients with idiopathic interstitial pneumonia.124
Figure 32-12 Interstitial lung disease. The patient presented with dyspnea and intermittent fever and the PET/CT showed diffusely increased FDG uptake in both lungs (arrows).
ROLE OF POSITRON EMISSION TOMOGRAPHY IN ASSESSMENT OF THE SOLITARY PULMONARY NODULE AND LUNG CANCER
Current clinical indications for FDG-PET imaging in malignant lung disease include evaluation of the solitary pulmonary nodule (SPN), staging of lung cancer (nonsmall cell, small cell, mesothelioma), and planning and monitoring the response to therapy.
 EVALUATION OF THE SOLITARY PULMONARY NODULE
EVALUATION OF THE SOLITARY PULMONARY NODULE
An SPN is defined as a round or oval radiographic opacity in the lung parenchyma that measures up to 3 cm in size and is not associated with mediastinal adenopathy or atelectasis (see Chapter 110).125 An SPN is commonly identified on chest radiographs or CT scans. In the United States, approximately 150,000 new SPNs are diagnosed per year; 30% to 50% of such lesions are malignant and may represent a potentially curable stage of bronchogenic carcinoma.126 Therefore, early and accurate diagnosis is essential for timely intervention, as lung cancer remains, by far, the leading cause of cancer death among both men and women in the world. Importantly, survival depends on the stage of the cancer when diagnosed.127
The most common type of lung cancer is nonsmall cell lung cancer (NSCLC) (adenocarcinoma, squamous cell carcinoma, and large cell carcinoma), which accounts for about 85% of lung cancers.127 About 10% to 15% of lung cancers are small cell carcinoma, which tend to spread quickly; fewer than 5% of lung cancers are neuroendocrine (carcinoid) tumors, which tend to grow slowly and rarely spread.127 Most malignant SPNs are adenocarcinoma (47%), followed by squamous cell carcinoma (22%), and small cell lung cancer.126 Of benign SPNs, about 80% are caused by infectious granulomas.125
While a number of benign etiologies for SPNs may have a characteristic appearance on CT, many cannot be characterized accurately using CT and often require further invasive assessment for accurate diagnosis. Certain factors, including smoking history, characteristics of the nodule (size, edge irregularity, and spiculation, pure ground-glass opacity, and absence of calcification), and the age when such lesions are detected are features that determine the probability of malignancy. Despite application of radiographic and other clinical criteria employed for distinguishing benign from malignant lesions, considerable overlap exists.128 CXRs and CT scans are not accurate in differentiating benign from malignant noncalcified pulmonary nodules that range from 1 to 3 cm in diameter. However, benign patterns of calcification and morphologic stability over 2 years are the most reliable signs of benignity.128 Bayesian analysis can be used to stratify risk (based on clinical information and imaging characteristics) and guide management of SPN.129
FDG-PET provides an accurate, noninvasive diagnostic assessment of SPNs, without the morbidity and costs associated with invasive tissue sampling.130 In a prospective multicenter trial designed to determine the utility of FDG-PET in discriminating between benign and malignant pulmonary nodules, the sensitivity and specificity of FDG-PET ranged from 92% to 98% and 69% to 100%, respectively (Table 32-6).130 False-positive studies are seen with active granulomas due to aspergillosis, tuberculosis, or sarcoidosis.106 False-positive findings may also be related to injection technique (Fig. 32-13). False-negative results are noted with hyperglycemia, malignancies that have a low metabolic activity (e.g., adenocarcinoma in situ, previously known as bronchoalveolar cell carncinoma, or carcinoid tumors),131 and nodules that are <8 mm in diameter. In one meta-analysis of 40 studies that included 1474 pulmonary lesions evaluated using FDG-PET (including dedicated and gamma camera-based PET), the technique had an average sensitivity of 97% and specificity of 78%.132