Periprocedural hyperglycemia is an independent predictor of mortality in patients who underwent percutaneous coronary intervention (PCI). However, periprocedural management of blood glucose is not standardized. The effects of routinely continuing long-acting glucose-lowering medications before coronary angiography with possible PCI on periprocedural glycemic control have not been investigated. Patients with diabetes mellitus (DM; n = 172) were randomized to continue (Continue group; n = 86) or hold (Hold group; n = 86) their clinically prescribed long-acting glucose-lowering medications before the procedure. The primary end point was glucose level on procedural access. In a subset of patients (no DM group: n = 25; Continue group: n = 25; and Hold group: n = 25), selected measures of platelet activity that change acutely were assessed. Patients with DM randomized to the Continue group had lower blood glucose levels on procedural access compared with those randomized to the Hold group (117 [97 to 151] vs 134 [117 to 172] mg/dl, p = 0.002). There were two hypoglycemic events in the Continue group and none in the Hold group, and no adverse events in either group. Selected markers of platelet activity differed across the no DM, Continue, and Hold groups (leukocyte platelet aggregates: 8.1% [7.2 to 10.4], 8.7% [6.9 to 11.4], 10.9% [8.6 to 14.7], p = 0.007; monocyte platelet aggregates: 14.0% [10.3 to 16.3], 20.8% [16.2 to 27.0], 22.5% [15.2 to 35.4], p <0.001; soluble p-selectin: 51.9 ng/ml [39.7 to 74.0], 59.1 ng/ml [46.8 to 73.2], 72.2 ng/ml [58.4 to 77.4], p = 0.014). In conclusion, routinely continuing clinically prescribed long-acting glucose-lowering medications before coronary angiography with possible PCI help achieve periprocedural euglycemia, appear safe, and should be considered as a strategy for achieving periprocedural glycemic control.
Periprocedural hyperglycemia predicts adverse events and mortality in patients who underwent percutaneous coronary intervention (PCI). Some data suggest that metabolic interventions during PCI may prevent deleterious effects of hyperglycemia. However, glycemic control has not been consistently achieved in trials using intravenous insulin, so not all trials have demonstrated a reduction in clinical events. There is wide variability in the management of long-acting glucose-lowering medications before coronary angiography and PCI in patients with diabetes mellitus (DM). Furthermore, in the current era of coronary angiography and PCI, where procedure times are shorter, sedation is minimal, and patients are able to eat shortly after the procedure; it is not certain if there is any need to routinely hold long-acting glucose-lowering medications before the procedure as done in surgical populations. Accordingly, in this randomized clinical trial, we sought to evaluate whether routinely continuing clinically prescribed long-acting glucose-lowering medications in patients with type 2 DM before coronary angiography with possible PCI effectively achieve periprocedural euglycemia. Because hyperglycemia is associated with increased platelet activity, we also explored the effect of continuing long-acting glucose-lowering medications on relevant measures of platelet activity.
Methods
All patients with type 2 DM referred for coronary angiography with possible PCI at the Manhattan Veterans Affairs Hospital were eligible to participate in the study. Patients were excluded if they were unable to give consent, were participating in a competing study, or had a history of symptomatic hypoglycemia ( Figure 1 ). All patients provided written, informed consent. This study was approved by the institutional review board and registered at ClinicalTrials.gov (identifier: NCT01419652 ).
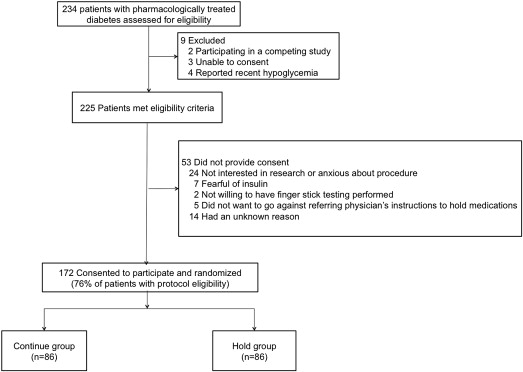
Randomization was performed in a 1:1 manner with sealed opaque envelopes using random block sizes of 4 and 8. Patients randomized to the Continue group were instructed to continue their clinically prescribed long-acting oral and injectable glucose-lowering medications as usual before the procedure. Patients prescribed metformin were instructed to continue their preprocedural dose as scheduled but not to resume therapy until 48 hours after the procedure per package insert. Patients randomized to the Hold group were instructed to hold all oral and injectable glucose-lowering medications the morning of the procedure (including glargine insulin the night before the procedure if prescribed). Blood glucose levels were not monitored routinely preprocedure, but only if patients expressed not feeling well. Upon procedural access, all patients had arterial blood collected through the sheath before any medication administration for assessment of blood glucose by point-of-care glucometer.
The primary end point was blood glucose level at the time of procedural access in the Continue versus Hold groups. The safety end point was the rate of hypoglycemic events (asymptomatic glucose <50 mg/dl or symptomatic glucose <75 mg/dl).
A platelet substudy (n = 75) was performed with 25 comparator patients with no history of DM, 25 patients from the Continue group, and 25 patients from the Hold group. Patients in the platelet substudy were either on aspirin therapy alone or on dual antiplatelet therapy with aspirin and clopidogrel before procedural access. Patients were excluded if they took cilostazol, dipyridamole, any nonsteroidal anti-inflammatory drugs other than aspirin, or any other antiplatelet agent as part of their prescribed medication regimen. Citrate-anticoagulated blood was collected at procedural access from the arterial sheath (minimum 5F) after an initial 2 cc discard for assessment of the following measures of platelet activity: monocyte platelet aggregate (MPA), leukocyte platelet aggregate (LPA) and soluble p-selectin. These markers of platelet activity were chosen based on their association with in vivo platelet activation, as well as their ability to change acutely and, thereby, may reflect acute changes in platelet activity caused by differences in glucose levels. MPA and LPA were measured through flow cytometery using directly conjugated CD14-PE, CD45-PE, and CD42a-fluorescein isothiocyanate antibodies. Measurements of soluble p-selectin were made using commercial enzyme-linked immunosorbent assay (eBioscience, San Diego, California).
Sample size was based on observational data in the catheterization laboratory at the Manhattan Veterans Hospital (mean glucose at procedural access in patients with DM of 154 ± SD 61 mg/dl) and an estimated decrease of mean glucose by 20% when continuing long-acting glucose-lowering medications. Glucose values on a log scale were normally distributed and used to calculate sample size (4.97 ± 0.36 mg/dl; estimated 3.2% decrease). Using a 2-sided 2-sample t test, approximately 82 patients with DM were needed in each group (Continue and Hold groups) to achieve 80% power at the 0.05 significance level (PASS 2008; NCSS, LLC., Kaysville, Utah).
Categorical variables are presented as proportions and compared using tests of proportions, whereas skewed continuous variables (Shapiro-Wilks test) are presented as median (interquartile range) and compared using Mann-Whitney and Kruskal-Wallis tests for 2- and 3-way comparisons. Confidence intervals for number of hyperglycemic events in each group were presented using the Wilson score interval. A significance of 0.05 was set for the primary comparison. Due to the exploratory nature of platelet activity testing and multiple measurements, a significance of 0.01 was set for comparisons of markers of platelet activity.
Results
Baseline characteristics of patients randomized to the Continue (n = 86) and Hold groups (n = 86) are listed in Table 1 . There were no major differences in baseline characteristics.
| Variable | Patients With DM | p Value | |
|---|---|---|---|
| Continue Group (n = 86) | Hold Group (n = 86) | ||
| Age (yrs) | 64 (60–74) | 66 (62–73) | 0.25 |
| Men | 85 (99) | 86 (100) | 1.0 |
| White | 62 (72) | 63 (73) | 0.58 |
| Black | 17 (20) | 13 (15) | |
| Hispanic | 7 (8) | 10 (12) | |
| Body mass index (kg/m 2 ) | 32 (29–36) | 30 (27–35) | 0.08 |
| Abdominal circumference (in) | 46 (42–50) | 44 (40–48) | 0.03 |
| Previous MI | 23 (27) | 26 (30) | 0.74 |
| Hypertension ∗ | 81 (94) | 81 (94) | 1.0 |
| Hyperlipidemia ∗ | 77 (90) | 78 (91) | 1.0 |
| Chronic kidney disease | 19 (22) | 13 (15) | 0.33 |
| Previous stroke | 15 (17) | 13 (15) | 0.84 |
| Peripheral vascular disease | 10 (12) | 17 (20) | 0.21 |
| Tobacco use | 27 (32) | 17 (20) | 0.11 |
| Presenting with acute coronary syndrome | 18 (21) | 25 (29) | 0.29 |
| Sulfonylurea | 37 (43) | 39 (45) | 0.88 |
| Metformin | 52 (61) | 53 (62) | 1.0 |
| Thiazoladinediones | 6 (7) | 4 (5) | 0.75 |
| Sitagliptin | 3 (4) | 0 | 0.25 |
| Long-acting insulin | 39 (45) | 35 (41) | 0.64 |
| Multiple glucose-lowering agents | 40 (47) | 35 (41) | 0.54 |
| Oral agents only | 15 (38) | 17 (49) | 0.36 |
| With insulin | 25 (63) | 18 (51) | |
| Ejection fraction | 0.64 | ||
| Normal | 64 (76) | 63 (74) | |
| Severely reduced | 6 (7) | 4 (5) | |
| Random glucose (mg/dl) | 141 (108–197) | 146 (120–186) | 0.55 |
| Hemoglobin A1c (%) | 7.1 (6.5–8.5) | 7.3 (6.8–8.0) | 0.94 |
| Glomerular filtration rate (ml/min) | 70 (54–84) | 72 (59–98) | 0.08 |
| Low-density lipoprotein cholesterol (mg/dl) | 74 (59–92) | 74 (61–94) | 0.66 |
| High-density lipoprotein cholesterol (mg/dl) | 39 (33–45) | 37 (32–45) | 0.96 |
| Triglyceride (mg/dl) | 151 (106–211) | 145 (105–214) | 0.81 |
| Fasting time (h) | 16.1 (14.0–19.2) | 17.3 (14.4–19.3) | 0.41 |
∗ Hypertension and hyperlipidemia are defined as self-reported history of or documentation of these diagnoses in a physician note in the electronic medical record system.
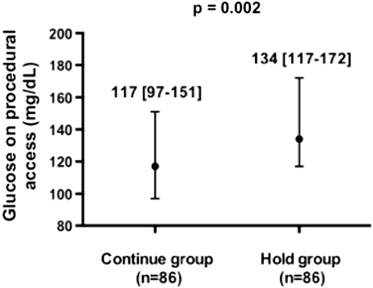
Blood glucose on procedural access was significantly lower in patients randomized to the Continue versus Hold groups ( Figure 2 ). There were 2 (0.01, 0.08) hypoglycemic events in the Continue group and 0 (0, 0.04) in the Hold group (p = 0.50). One event was an asymptomatic blood glucose of 43 mg/dl on procedural access, treated with intravenous glucose. This patient was on long-acting insulin, metformin, and glyburide before angiography. The second event was a blood glucose of 48 mg/dl 3 hours before the procedure associated with perspiration and treated successfully with orange juice. This patient was on long-acting insulin, metformin, and glipizide before the procedure and had a glucose of 167 mg/dl on procedural access.
Baseline characteristics of the patients enrolled in the platelet substudy are listed in Table 2 . As expected, median glucose at the time of procedural access was higher in the Hold compared with Continue and without DM groups ( Figure 3 ). Platelet activity at the time of procedural access, as demonstrated by LPA, MPA, and soluble p-selectin levels, was also higher in the Hold compared with Continue and no DM groups ( Figure 3 ).
| No DM (n = 25) | Continue Group (n = 25) | Hold Group (n = 25) | p Value ∗ | p Value † | |
|---|---|---|---|---|---|
| Age (yrs) | 65 (61–77) | 65 (63–71) | 68 (63–73) | 0.52 | 0.28 |
| Men | 23 (92) | 25 (100) | 25 (100) | 0.13 | 1.0 |
| White | 20 (80) | 19 (76) | 20 (80) | 0.38 | 0.34 |
| Black | 5 (20) | 4 (16) | 5 (20) | ||
| Hispanic | 0 | 2 (8) | 0 | ||
| Body mass index (kg/m 2 ) | 29 (27–32) | 34 (30–36) | 30 (27–33) | 0.03 | 0.04 |
| Abdominal circumference (in) | 40 (37–44) | 47 (44–52) | 44 (39–47) | 0.001 | 0.03 |
| Previous MI | 5 (20) | 10 (40) | 7 (28) | 0.30 | 0.55 |
| Hypertension | 20 (80) | 24 (96) | 22 (88) | 0.22 | 0.61 |
| Hyperlipidemia | 18 (72) | 22 (88) | 24 (96) | 0.05 | 0.61 |
| Chronic kidney disease | 2 (8) | 5 (20) | 5 (20) | 0.41 | 1.0 |
| Previous stroke | 3 (12) | 4 (16) | 2 (8) | 0.68 | 0.67 |
| Peripheral vascular disease | 4 (16) | 2 (8) | 4 (16) | 0.63 | 0.67 |
| Tobacco use | 7 (28) | 5 (20) | 5 (20) | 0.74 | 1.0 |
| Presenting with acute coronary syndrome | 5 (20) | 5 (20) | 7 (28) | 0.74 | 0.74 |
| Dual antiplatelet therapy with aspirin and clopidogrel before arterial access | 14 (56) | 13 (52) | 16 (64) | 0.80 | 0.65 |
| Sulfonylurea | — | 11 (44) | 12 (48) | — | 1.0 |
| Metformin | — | 16 (64) | 16 (64) | — | 1.0 |
| Thiazoladinediones | — | 2 (8) | 0 | — | 0.49 |
| Sitagliptin | — | 2 (8) | 0 | — | 0.49 |
| Long-acting insulin | — | 9 (36) | 10 (40) | — | 1.0 |
| Ejection fraction | 0.33 | 0.18 | |||
| Normal | 18 (72) | 16 (67) | 21 (88) | ||
| Severely reduced | 2 (8) | 7 (29) | 1 (4) | ||
| Random glucose (mg/dl) | 90 (83–96) | 141 (109–168) | 144 (116–182) | <0.001 | 0.67 |
| Hemoglobin A1c (%) | 5.8 (5.5–6.0) | 7.1 (6.4–7.9) | 7.0 (6.8–7.8) | <0.001 | 0.78 |
| Platelets (×10 9 /L) | 198 (139–236) | 193 (171–244) | 194 (169–228) | 0.84 | 0.85 |
| Mean platelet volume (fl) | 9.1 (8.5–9.7) | 8.7 (7.7–9.3) | 9.0 (7.9–9.6) | 0.34 | 0.52 |
| Glomerular filtration rate (ml/min) | 90 (69–140) | 80 (54–89) | 72 (57–96) | 0.19 | 0.69 |
| Low-density lipoprotein cholesterol (mg/dl) | 91 (70–140) | 71 (55–86) | 69 (56–82) | 0.008 | 0.98 |
| High-density lipoprotein cholesterol (mg/dl) | 41 (36–45) | 38 (31–46) | 35 (32–51) | 0.28 | 0.58 |
| Triglyceride (mg/dl) | 133 (85–199) | 138 (109–197) | 154 (106–186) | 0.84 | 0.96 |
| Number of coronary arteries narrowed | |||||
| None | 8 (32) | 5 (20) | 7 (28) | 0.33 | 0.33 |
| 1 vessel | 6 (24) | 8 (32) | 3 (12) | ||
| 2 vessel | 3 (12) | 8 (32) | 8 (32) | ||
| 3 vessel | 8 (32) | 4 (16) | 7 (28) | ||
| Fasting time (h) | 17.8 (15.1–18.9) | 16.0 (13.5–18.5) | 16.8 (15.6–18.2) | 0.45 | 0.36 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


