CHAPTER 74 Peripheral Angiography and Percutaneous Intervention
Peripheral arterial occlusive disease (PAD) is a major cause of disability, loss of work, and lifestyle changes. The natural history of PAD is often one of slow progression in symptoms over time.1–5 However, 70% of patients will remain stable or improve over time, approximately 30% will require an intervention, and 10% will require amputation.6 Limb loss is the tragic final outcome of PAD and is associated with a significant degree of disability and a poor overall prognosis.7 The increasing age of the population and continued tobacco abuse, high-fat diets, sedentary lifestyles, and rising prevalence of obesity parallel the increasing prevalence of PAD. The prevalence of PAD in the general population is 3% to 10%, and it is 15% to 20% among patients older than 70 years.8 This probably underestimates the true burden of disease, however, as PAD may be asymptomatic. Because of the rising prevalence of PAD, many cardiovascular specialists have adopted a strategy of “global vascular management” for their patients. In this strategy, the evaluation, care, and intervention plan is more comprehensive in its scope and includes the management of vascular disease in all of its manifestations and anatomic locations. To this end, we review here the general aspects of peripheral vascular disease with particular emphasis on the angiographic and percutaneous interventions after medical evaluation.
PERIPHERAL ANGIOGRAPHY
Indications
The purpose of peripheral angiography is to define the vascular anatomy and to identify significant arterial narrowing that requires revascularization (either percutaneous or surgical). As with any invasive diagnostic test, the risk-benefit ratio should be evaluated before angiography to identify those patients who might fully benefit from imaging.9–11
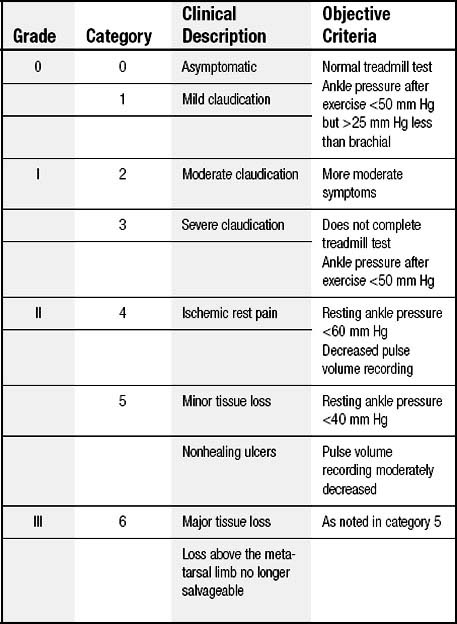
Lower extremity claudication is classified according to the level of debilitation or restriction of activities. It is important to select the appropriate patient for angiography, given that the morbidity from vascular access may be particularly high among patients with PAD. The most commonly used clinical grading system for PAD is the Rutherford-Becker scale, a 7-point scale from 0, which denotes asymptomatic disease, to 6, which denotes major tissue loss (Table 74-1). In general, patients with Rutherford scale 2 and higher and noninvasive studies that demonstrate evidence of significant disease are likely to benefit from angiography and revascularization. Any patient with symptomatically limiting lower extremity claudication should undergo angiography to fully define the anatomy with an eye toward revascularization.
Angiography is indicated in the patient with threat of limb loss due to acute thromboembolism. Patients with acute onset of a cool, pulseless extremity after catheterization or trauma require angiography to define the anatomy and site of occlusion. Angiography may allow the direct infusion of intra-arterial fibrinolytic therapy or mechanical or rheolytic thrombectomy.12–17
Invasive renal angiography is indicated in patients who have evidence of significant stenosis on noninvasive testing, such as magnetic resonance angiography and Doppler ultrasonography. Other indications include new uncontrolled hypertension in patients younger than 30 years or older than 55 years, refractory hypertension, rising creatinine concentration (specifically after the institution of angiotensin-converting enzyme inhibitor therapy), or unexplained acute pulmonary edema.18 The prevalence of renovascular hypertension may be as high as 5% in the general population and 30% among patients with coronary artery disease or other PAD.
Because of the multiple-source blood supply to the upper extremity, which is derived principally from the subclavian and vertebral arteries, upper extremity claudication is rare. Upper extremity claudication symptoms include an inability to do activities with the affected limb, such as combing hair, brushing teeth, or repeated lifting. Another constellation of symptoms indicating obstructive disease in the upper extremity may come from significant obstruction in the posterior circulation. In this situation, there is a reversal of flow from the vertebral artery in a patient with disease in the ipsilateral vertebral artery, leading to dizziness or difficulties with gait.19–22 Also, among patients who have undergone coronary artery bypass graft surgery with use of the internal mammary artery, persistent angina and anterior ischemia on noninvasive testing may suggest left subclavian stenosis as the cause of coronary ischemia.21,23–27 Therefore, any patient with symptoms of upper extremity claudication, anterior ischemia after coronary artery bypass grafting with the use of the left internal mammary artery, or posterior circulation events and a difference in upper extremity blood pressures should be evaluated for the possibility of subclavian stenosis and undergo evaluation, including angiography.
Contraindications
The principal contraindications to peripheral angiography are bleeding diathesis, renal failure (true or impending), fever, ongoing infection, and severe anemia (Table 74-2).
Table 74–2 Relative Contraindications to Catheterization and Angiography
| Bleeding diathesis or inability to take aspirin or adenosine diphosphate inhibitor |
| Concurrent febrile illness |
| Severe renal insufficiency or anuria without dialysis planned |
| Severe allergy to contrast agents |
| Severe hypokalemia or digitalis toxicity |
| Severe hypertension or ongoing unstable coronary syndrome |
Complications
Complications of invasive peripheral angiography primarily involve vascular access site complications, catheter manipulation within atherosclerotic vessels, emboli, clot formation, stroke, myocardial infarction, worsening renal function, or congestive heart failure (Table 74-3). A particularly devastating complication of catheter manipulation within the arterial tree is an atheroembolic event. In its most severe form to the lower extremities, it may cause loss of limb or digits. Likewise, emboli to the renal circulation may lead to acute renal decompensation or failure requiring dialysis.28–30 Pseudoaneurysm or vascular access complications may be as high as 3% in patients with severe peripheral occlusive disease.31,32
Table 74–3 Possible Complications of Peripheral Catheterization
| Vascular access dissection or perforation | 0.1%-0.2% |
| Bleeding or hematoma | 1.5%-2.0% |
| Allergic reaction | 0.5%-2.0% |
| Vasovagal events | 1.0%-2.0% |
| Death | 0.1%-0.2% |
General Considerations
There are several important differences in imaging of peripheral vascular structures and coronary arteries. Peripheral angiography commonly employs a larger image intensifier field (14 inches or 36 cm) to encompass larger regions of interest. Digital (not film-based) angiography allows online display of acquired images as well as advanced processing techniques to enhance brightness, contrast, or shift of underlying bone structures to enhance the final image (Fig. 74-1). Frequently, digital subtraction angiography is used. With this imaging method, a baseline background image, referred to as a mask, is obtained immediately before injection of contrast material to digitally subtract bone, calcifications, air, or soft tissues from the final image, leading to the best image quality with the most definition of vascular anatomy. Another advantage of digital subtraction angiography is that it may reduce the volume of contrast material and imaging acquisition time required.
SPECIFIC ANATOMIC IMAGING
Aorta
Thoracic Aorta
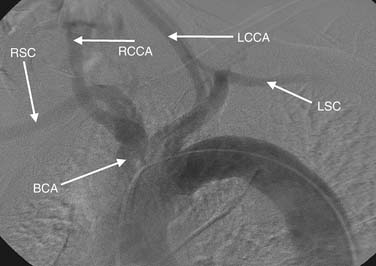
Thoracic aortography is used to define the anatomic relation of the aortic arch and the great vessels, to determine the diameter of the thoracic aorta, to obtain evidence of dissection (Fig. 74-2), and to evaluate for trauma or other vascular injuries. The optimal view of the aortic arch is obtained in the left anterior oblique projection at 30 to 40 degrees oblique. The injection of 30 to 40 mL of contrast material at 20 to 30 mL/sec generally opacifies the vessels for adequate imaging. If digital subtraction angiography is used, the patient should be instructed to hold the breath to avoid artifacts due to motion or breathing. The three principal vessels originating from the arch are (1) the brachiocephalic (innominate), leading ultimately to the right subclavian and right carotid arteries; (2) the left common carotid; and (3) the left subclavian artery (Fig. 74-3). The most common normal variant of this anatomy is the so-called bovine arch, in which the left common carotid artery originates from the brachiocephalic trunk. This normal variant occurs in approximately 10% of patients.32
Abdominal Aorta
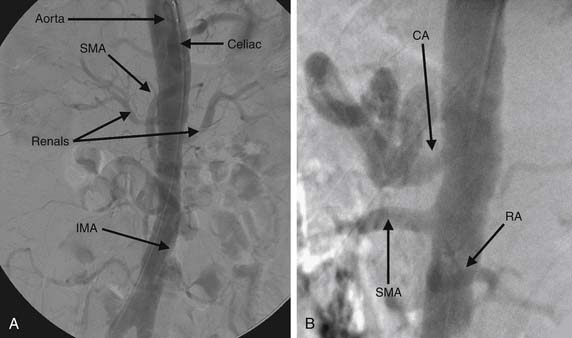
In general, 20 to 40 mL of contrast material injected at 15 to 30 mL/sec will adequately opacify the vessels to define the anatomy. Again, as in the thoracic aorta, it is critical to fully opacify the lumen and the vessels of interest. AP and lateral images (Fig. 74-4) of the abdominal aorta are usually sufficient to delineate all anatomic areas of interest in the abdominal aorta and mesenteric vessels. If selected conduits require further visualization, a soft-tipped catheter may provide selective angiography without requiring large loads of contrast material to fully define the anatomy.
Head and Neck Angiography
The three primary branches emanating from the aortic arch are the brachiocephalic, left common carotid, and left subclavian arteries. The right subclavian and carotid arteries are the terminal branches of the brachiocephalic trunk. The most common variant of this anatomy is the bovine arch, in which the left carotid and brachiocephalic share a common origin.32
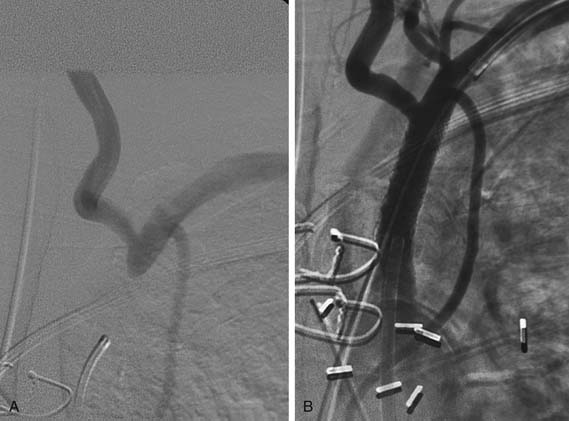
Subclavian artery disease, stenosis, and occlusion are common.33 Symptoms are rare because of the dual supply of blood to the upper extremity through the subclavian and vertebral arteries. Upper extremity claudication is rare but is manifested by upper extremity discomfort while engaging in activity with the upper extremity or in activities of daily living. More commonly, subclavian stenosis or occlusion is manifested by either posterior circulation symptoms, such as dizziness and gait instability, which are rare, or anterior ischemia (coronary steal syndrome) in the patient who has been treated with left internal mammary artery coronary bypass grafting (Fig. 74-5).23–2734
The carotid arteries generally bifurcate at the level of the fourth cervical vertebra into the internal and external carotid arteries (Fig. 74-6A). The internal carotid usually has no major branches and becomes tortuous below the petrous bone, the so-called carotid siphon. Once the vessel enters the petrous bone, it is considered the intracranial internal carotid artery. On exiting the petrous bone, the internal carotid artery bifurcates early into the anterior and middle cerebral arteries. The external carotid artery has several branches that supply the face.
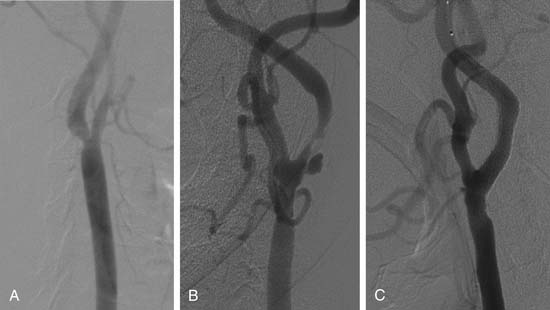
Carotid atherosclerosis is common.35,36 Intervention in the internal carotid artery is performed either percutaneously or surgically. Annually, there are 600,000 strokes in the United States, of which 500,000 are first attacks and 160,000 are fatal. Cerebral occlusive disease encompasses stroke, transient ischemic attack, and posterior circulation (vertebral-basilar) events. Several studies have evaluated the efficacy of various revascularization strategies in preventing future events.37–39 The National Heart, Lung, and Blood Institute’s Atherosclerosis Risk in Communities study40 reported that 83% of all strokes were ischemic, 40% were lacunar, and 14% were thromboembolic. Most patients with extracranial carotid artery disease are asymptomatic.
Risk factors for the presence and progression of carotid artery disease are similar to those of coronary artery disease and include diabetes mellitus, hypertension, hyperlipidemia, and family history. A bruit may aid in identifying patients with carotid stenoses, but the presence of a bruit is neither sensitive nor specific for the detection of clinically meaningful carotid stenosis. For example, in one study,41 only 37% of 330 patients referred to a neurology clinic with a cervical bruit had a high-grade carotid artery lesion noted with duplex imaging. A carotid bruit may be associated with subsequent risk of stroke, as the Framingham Heart Study demonstrated that asymptomatic patients with a carotid bruit have twice the risk of stroke.42 In addition, a bruit may be a marker of more general cardiovascular risk.43 In asymptomatic patients, risk of stroke was 2.5% if the stenosis was more than 75%. The risk was higher for patients with symptoms (3.3%).44–46
Renal Vascular Imaging
The prevalence of renovascular disease may be as high as 50% in patients with coronary artery disease or other PAD.47,48 Renovascular disease is a cause of secondary hypertension in 0.5% to 5% of the general population.49,50 Whereas other noninvasive and functional studies such as Doppler ultrasonography, captopril nuclear studies, and magnetic resonance angiography remain vital screening tools, contrast angiography remains the “gold standard.” The renal arteries have a posterior takeoff from the abdominal aorta. Injection of 15 to 20 mL of contrast material at 30 mL/sec should opacify the intended vessels adequately.
An improvement in blood pressure control is one goal of percutaneous revascularization of renal arteries.51–53 Another potential benefit of renal artery revascularization is preservation of renal function.48,54–56
Lower Extremity Angiography
Iliac Vessels
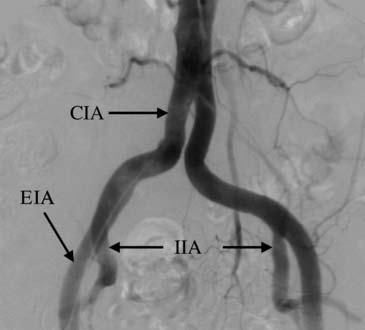
The iliac vessels originate at the termination of the abdominal aorta, usually at the L4-5 level. They remain retroperitoneal throughout their course until they cross the inguinal ligament and become the common femoral artery. The principal bifurcation in the iliac artery is the terminal bifurcation of the common iliac into the internal and external iliac arteries (Fig. 74-7).
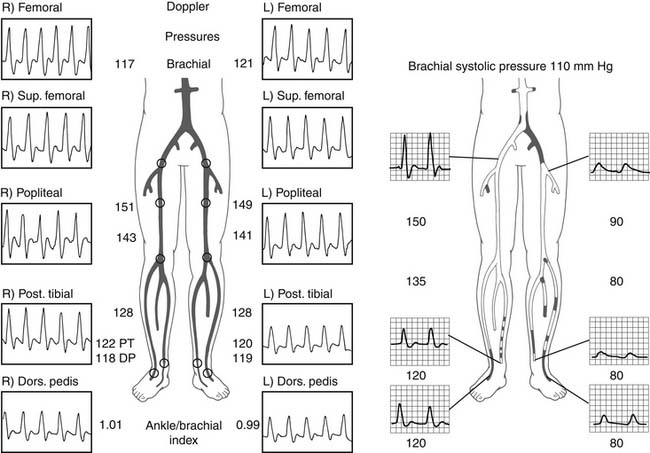
In patients with lower extremity claudication, noninvasive testing with ankle-brachial indices by duplex and Doppler imaging is critical. Evaluation with and without exercise, either 5 minutes at 1.5 miles per hour or 40 calf raises,57 is often necessary to provoke symptoms and, more important, to determine the level of stenosis. Doppler imaging is vital in the evaluation of the lower extremities. Normal flow patterns are triphasic or biphasic (Fig. 74-8). When a critical lesion is present, the waveform becomes monophasic (see Fig. 74-8). Furthermore, during exertion, resting triphasic and biphasic waveforms become monophasic distal to a critical stenosis. The location of the transition in the waveform pattern indicates the level of critical disease and can allow more directed and focused lower extremity angiography. Because the lower extremity arterial tree is relatively superficial, the anatomy can occasionally be directly visualized noninvasively with duplex imaging. Therefore, in some cases, the level and extent of disease may be delineated without vascular access.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree