Perioperative Respiratory Considerations in the Surgical Patient
Postoperative pulmonary complications constitute a significant cause of morbidity and mortality following surgery. Managing patients at risk for postoperative pulmonary problems requires an understanding of the predictable changes in pulmonary physiology that occur with surgery and anesthesia, as well as knowledge of factors associated with development of postsurgical respiratory compromise. Despite the availability of several screening tests, a careful history and physical examination continue to be the cornerstone of preoperative pulmonary evaluation. Although a number of measures can be employed before and after surgery to minimize the risk of respiratory complications, close patient monitoring and early detection are essential.
This chapter focuses initially on changes in pulmonary function with surgery. Pulmonary risk factors before, during, and after surgery are reviewed prior to discussion of preoperative evaluation of the patient for surgery, including lung resectional surgery. Finally, recommendations are made regarding preoperative preparation and postoperative prophylactic measures. A more detailed discussion of the perioperative care of the patient undergoing resectional lung surgery is provided in Chapter 105 and development of acute respiratory failure in the surgical patient is addressed in Chapter 104.
CHANGES IN PULMONARY FUNCTION WITH SURGERY
Many postoperative respiratory complications relate to exaggerations of the expected postoperative changes in pulmonary function that occur as a result of the surgery itself, anesthesia, or various pharmacologic interventions.1,2 Hence, an appreciation of normal postoperative pulmonary physiology is useful in understanding a number of pulmonary problems seen following surgery. Five principal categories of change in pulmonary function with surgery may be considered: (1) lung volumes, (2) diaphragm function, (3) gas exchange, (4) control of breathing, and (5) lung defense mechanisms (Table 103-1).
Source: Reproduced with permission from Goldmann DR, Brown FH, Guarnieri DM (eds). Perioperative Medicine. New York, McGraw-Hill; 1994.
 LUNG VOLUMES
LUNG VOLUMES
The pattern of pulmonary function abnormalities following thoracic and abdominal surgery is restrictive, characterized by moderate-to-severe reductions in vital capacity (VC) and smaller, but more important, reductions in functional residual capacity (FRC). The degree of impairment is similar after upper abdominal and thoracic surgery3–5 and is less for laparoscopic procedures compared with open abdominal procedures.6 Smaller changes in VC and FRC are noted with lower abdominal surgery; superficial or extremity surgery is usually not associated with any significant or persistent changes in lung volumes.7,8 During the first 24 hours following upper abdominal surgery, VC and FRC may be reduced by more than 70% and 50%, respectively, and they may remain depressed for more than a week.7–9 Consequently, it is not surprising that pulmonary complications are seen more often with thoracic and upper abdominal procedures than with surgery involving the lower abdomen or extremities (see discussion of intraoperative risk factors, below).
Reductions in other lung volumes, including total lung capacity (TLC), inspiratory capacity (IC), expiratory reserve volume (ERV), and residual volume (RV) have been noted. While the forced expiratory volume in 1 second (FEV1) is decreased, the ratio of FEV1 to the forced vital capacity (FEV1/FVC%) remains unchanged, indicating that major airway obstruction does not occur.4
Since patients undergoing superficial or extremity surgery do not experience major changes in lung volumes, residual or carryover effects from general anesthesia do not appear to play a primary role in this regard. In fact, studies have shown that in many patients, FRC in the early postoperative period is unchanged from baseline. An alternative proposal for the reduction in FRC is that postsurgical pain and associated muscle splinting impair lung mechanics. However, while processes other than pain may be operative, based on the finding that effective pain control using epidural analgesia or intercostal nerve block fails to fully restore VC or FRC to preoperative levels,10–12 this notion has been challenged.13 The established consensus is that diaphragm dysfunction is an important contributing factor (see below).2
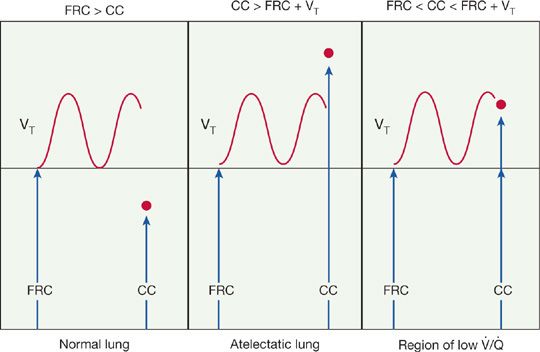
The postoperative reduction in FRC is of major physiologic significance. Its importance can be understood when the phenomenon of airway closure and the concept of closing capacity (CC) are considered.4
FRC is the lung volume at the end of a normal tidal expiration. CC is the lung volume at which small airways in the lung bases begin to close during expiration because of a reduction in airway radial traction. The relationship between the two is a key factor in the development of postoperative changes in lung function (Fig. 130-1). In a normal lung, FRC is always greater than CC, and the airways remain open throughout a tidal breath. However, when CC is greater than FRC, lung volume fails to increase sufficiently during tidal breathing to open all the airways and, consequently, some alveolar units remain closed during a breath. Such regions constitute areas of atelectasis. An intermediate state exists when CC exceeds lung volume for part of the time during each tidal breath. Under these circumstances, the airways open for only a portion of the respiratory cycle, creating areas of low ventilation relative to perfusion. In summary, any circumstance that reduces FRC below CC or that increases CC above FRC produces regions of reduced ventilation and atelectasis (Table 103-2).
Figure 103-1 The relationship between functional residual capacity (FRC) and closing capacity (CC). VT = Tidal volume. See text. (Reproduced with permission from Goldmann DR, Brown FH, Guarnieri DM (eds). Perioperative Medicine. New York, McGraw-Hill; 1994.)
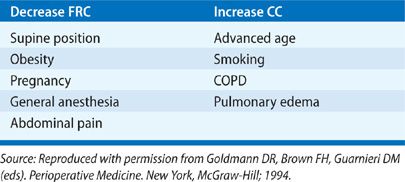
TABLE 103-2 Conditions that Alter the Relationship between Functional Residual Capacity (FRC) and Closing Capacity (CC)
 DIAPHRAGM FUNCTION
DIAPHRAGM FUNCTION
Diaphragm dysfunction has been recognized as an important factor contributing to the postoperative reduction in lung volumes.6,11–16 In patients undergoing cholecystectomy, the diaphragm’s contribution to quiet tidal breathing after surgery is reduced.14 Measurements of transdiaphragmatic pressure during maximal phrenic nerve stimulation following upper abdominal surgery indicate that decreased central nervous system output to the phrenic nerves, possibly as a result of inhibitory reflexes arising from sympathetic, vagal, or splanchnic receptors, may be the important etiologic factor.15 Studies of the role of pain in this impairment have yielded conflicting results, pointing to either a limited10–12 or significant13 role for pain, depending on the procedures used for analgesia.
 GAS EXCHANGE
GAS EXCHANGE
Postoperative arterial hypoxemia occurs commonly, and two phases in its development may be described.17,18
The initial phase occurs in the first several hours following anesthesia and surgery. The underlying mechanisms are related largely to the residual effects of the anesthesia and include ventilation–perfusion mismatch, anesthetic-induced inhibition of hypoxic pulmonary vasoconstriction, right-to-left shunting, alveolar hypoventilation, depressed cardiac output, and increased oxygen consumption by peripheral muscles. This phase resolves within 24 hours following superficial surgery.
A second phase of hypoxemia, which may persist for several days or weeks, is seen after thoracic and upper abdominal surgery. This phase correlates with reductions in FRC and changes in the FRC–CC relationship. Although alterations in the FRC–CC relationship predominate, other processes may contribute to late postoperative hypoxemia: (1) alveolar hypoventilation (see discussion of control of breathing below); (2) increased dead space ventilation due to rapid, shallow breathing; and (3) decreased mixed venous oxygen tension due to increased oxygen consumption, impaired cardiac output, and reduced oxygen carrying capacity.
 CONTROL OF BREATHING
CONTROL OF BREATHING
Respiratory depression is a common feature of the postoperative period. Two factors are responsible. First, residual effects of preanesthetic or anesthetic agents inhibit respiratory drive and reduce the ventilatory response to hypercapnia, hypoxia, and acidosis.19 Second, narcotics given for postoperative analgesia depress both hypercapnic and hypoxic ventilatory drives, resulting in decreased tidal volume, reduced minute ventilation, and increased PaCO2.20 Narcotics also alter the pattern of breathing, reducing the frequency of sighs or eliminating them entirely; in susceptible patients, narcotics may precipitate sleep apnea.
 LUNG DEFENSE MECHANISMS
LUNG DEFENSE MECHANISMS
Several mechanisms protect the lung from environmental and infectious insults. Two of the most important – cough and mucociliary transport – are compromised after surgery, contributing to an increased risk of pulmonary infection.4,21 Postoperative pain or excessive use of narcotics may inhibit coughing; in addition, altered lung mechanics decrease the expulsive force generated with each cough. Mucociliary clearance is impaired for up to a week following upper abdominal surgery. Although an ineffective cough reflex contributes significantly to reduced mucociliary clearance, several additional mechanisms are involved. These include (1) cilia damage from endotracheal intubation and inhalation of dry, hyperoxic gas mixtures; (2) reduced tracheal mucus velocity due to the presence of an endotracheal tube; (3) anesthetic-induced inhibition of mucociliary transport; and (4) atelectasis.
PULMONARY COMPLICATIONS
The criteria used for defining postoperative pulmonary morbidity have varied considerably in published reports,22,23 although it is clear that, from a broad perspective, five major categories of complications may be considered: (1) atelectasis; (2) infection, including acute tracheobronchitis and pneumonia; (3) exacerbation of underlying chronic lung disease; (4) prolonged mechanical ventilation and respiratory failure; and (5) thromboembolic disease. With thoracic surgery, several additional problems may occur,24–36 which are summarized in Table 103-3.
TABLE 103-3 Pulmonary Complications Associated with Thoracic Surgery

The variability in defining postoperative pulmonary complications has resulted in reported incidences that have ranged widely in the literature, depending on the patient population sampled and the procedures studied. In general, a healthy, young nonsmoker of normal weight has a very low risk of postoperative pulmonary complications (1% or less).37 However, a number of factors have been identified that are associated with the development of postoperative pulmonary complications (Table 103-4).22,23,38–41 They include preoperative factors (chronic lung disease, smoking, general state of health, age, obesity, nutritional status, and antecedent respiratory tract infection), intraoperative factors (the emergent nature of the procedure, type and duration of anesthesia, location of surgical site, and type of surgical incision), and postoperative factors (inadequate pain control and immobilization). Tables 103-5 and 103-6 list, respectively, the relative strengths of preoperative and intraoperative factors associated with development of postoperative pulmonary complications based on odds ratios.23,42
TABLE 103-4 Factors Associated with Development of Postoperative Pulmonary Complications
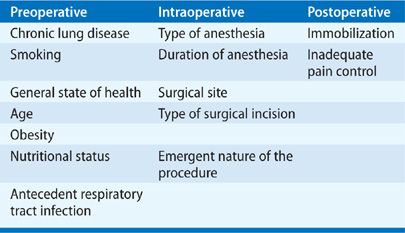
TABLE 103-5 Relative Strength of Preoperative Risk Factors Associated with the Development of Postoperative Pulmonary Complications
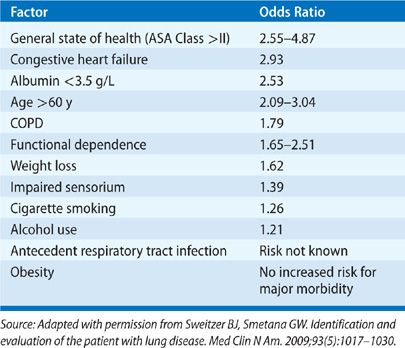
TABLE 103-6 Relative Strength of Intraoperative Risk Factors Associated with the Development of Postoperative Pulmonary Complications
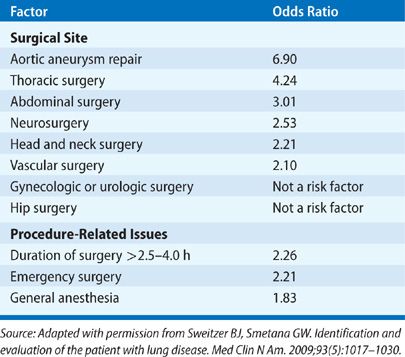
PREOPERATIVE RISK FACTORS
A number of patient-related factors have been implicated in the development of postoperative respiratory complications.23 They include the presence of chronic lung disease (particularly obstructive airway disease); the patient’s overall state of health, age, and history of cigarette smoking; and the presence of comorbid conditions, including malnutrition, congestive heart failure, alcohol use, functional dependence, and impaired sensorium. The precise risk associated with recent respiratory viral infections is unknown. Obesity appears not to increase the risk for major postoperative pulmonary complications.
 CHRONIC LUNG DISEASE
CHRONIC LUNG DISEASE
The following discussion focuses on the operative risks in patients who have one of four common categories of chronic lung disease: (1) chronic obstructive pulmonary disease (COPD), (2) restrictive lung diseases, (3) obstructive sleep apnea (OSA), and (4) pulmonary vascular diseases.
Chronic Obstructive Pulmonary Disease
Most studies addressing the impact of pre-existing lung disease on surgical risk have focused on COPD, which has been identified as a significant risk for development of postoperative pulmonary complications.38,39,43–53 The reported incidence of postoperative pulmonary complications in patients with COPD varies from 10% to greater than 50% and is influenced by type of surgery, magnitude of pre-existing respiratory impairment, and criteria used to define complications. Although not precisely quantified in the literature, the risk for postoperative respiratory complications appears to increase significantly when the FEV1 is below 65% of predicted. Evidence exists that the risk for postoperative respiratory complications in COPD may be raised further by the concomitant presence of resting hypoxemia.54 Although an increased risk has been suggested for patients who are hypercapnic at rest,43,55 whether hypercapnia is, in fact, an independent predictor of pulmonary complications after surgery is unclear.56
In patients with severe disease, an important issue is whether a critical level of lung function exists below which the risk of developing a major, potentially life-threatening pulmonary complication is so high as to make anesthesia and surgery too dangerous. In the past, such a prohibitive threshold or level was proposed. Subsequent studies, however, have failed to support this hypothesis.57 Patients with an FEV1 as low as 450 mL have been found to tolerate surgery safely.55 Hence, patients should not be denied necessary operative procedures solely on the basis of marginal lung function. As with all medical interventions, the potential benefits of the operative procedure must be weighed against the operative risk.
The increased incidence of postoperative pulmonary complications in patients with COPD is due, in part, to an increase in the CC, favoring development of areas of low ventilation-to-perfusion ratios and atelectasis. In addition, in patients who continue to smoke, impaired ciliary function and chronic tracheobronchitis may be contributing factors.
Restrictive Lung Diseases
The risk of pulmonary complications in patients with restrictive lung diseases who undergo surgery is unknown. Although some experience has been reported with patients having thoracic or corrective orthopedic surgery (see below), very little data exist with regard to abdominal and extremity surgery. One might expect a higher incidence of postoperative respiratory complications in these patients for two reasons: (1) FRC is reduced, favoring the formation of areas of poor ventilation and atelectasis; (2) coughing and the ability to clear respiratory secretions are impaired.
Experience with postoperative pulmonary complications has been reported in three relatively common situations for patients with restrictive disorders: (1) sarcoidosis complicated by aspergilloma and hemoptysis, (2) corrective surgery for kyphoscoliosis, and (3) myasthenia gravis with associated thymoma.
Sarcoidosis may progress to diffuse interstitial fibrosis and cavitary changes, primarily involving the upper lobes (see Chapter 55, Moller). The cavities are prone to infection with Aspergillus species and aspergilloma formation, with subsequent development of recurrent and, at times, life-threatening, hemoptysis. Affected patients generally have very poor lung function and, hence, are managed conservatively. However, if supportive medical therapy fails, patients may require thoracotomy and lung resection. These procedures can be done with low mortality, but they may be complicated by empyema, chylothorax, prolonged pulmonary parenchymal air leaks, or bronchopleural fistulae.30–32
Corrective surgery in patients with kyphoscoliosis may involve anterior or posterior spinal fusion procedures or a combination of the two.58,59 In addition to correction of the primary orthopedic abnormality, an important indication for performing these procedures is progressive deterioration of pulmonary function. Postoperative respiratory complications have been reported in up to 20% of patients, including pleural space–related processes (e.g., pneumothorax, pleural effusion, bronchopleural fistula, and empyema) and lobar or total lung atelectasis.59–61 Important risk factors include (1) nonidiopathic scoliosis, (2) open anterior spinal fusion procedures, (3) age greater than 20 years, (4) mental retardation, (5) preoperative hypoxemia, and (6) obstructive pulmonary function tests.60 Thoracotomy has been associated with a significant decrease in pulmonary function for up to 2 years after surgery.59,62 Video-assisted thoracoscopic surgery (VATS) has emerged as an alternative to open thoracotomy; the outcomes of anterior fusion via VATS and thoracotomy are similar.63,64
The majority of patients with myasthenia gravis will, during the course of their disease, undergo thymectomy, which appears to improve long-term outcomes.65 Surgical options for thymectomy include the long-established method of transsternal resection, a transcervical approach, a combined transsternal–transcervical procedure, and, more recently, VATS.66,67
Historically, up to 30% of patients undergoing thymectomy required mechanical ventilation for more than 3 days following the transsternal approach.68,69 Reported risk factors for postoperative pulmonary complications following transsternal resection include chronic myasthenia gravis (>6 years), severe bulbar weakness, pre-existing respiratory illness, need for large doses of pyridostigmine, and reduced maximal static expiratory pressure (<50 cm H2O or 66% of predicted).68–70 Preoperative VC has not proved a consistent predictor of respiratory morbidity following thymectomy. More recent routine use of plasma exchange in patients with bulbar or generalized myasthenia gravis has significantly reduced the duration of postoperative ventilatory support and time in the intensive care unit following transsternal thymectomy.66
Video-assisted thoracoscopic thymectomy is an increasingly common approach, with outcomes equivalent or superior to more invasive procedures.67
Virtually all patients with myasthenia gravis are treated with anticholinesterases, which are usually discontinued prior to surgery to minimize tracheobronchial secretions. Controversy exists, however, regarding whether these agents should be restarted immediately after surgery or withheld for 24 to 48 hours following thymectomy.
Obstructive Sleep Apnea
OSA is common, with a prevalence of 5% or higher, depending on the population studied (see Chapter 99, Pien and Rosen). For many of these patients, the diagnosis of OSA is not established preoperatively.71 Unrecognized OSA is significant, because both anesthesia and surgery affect sleep architecture; in addition, use of postoperative narcotics for analgesia may further blunt ventilatory chemosensitivity, suggesting that postoperative respiratory morbidity is likely higher in patients with OSA than in the general population. Although initial studies were consistent with this expectation,72 subsequent reports have failed to show that OSA is significant risk factor for development of major postoperative pulmonary complications.73–75 Thus, while it is prudent and in the patient’s best interest to use the preoperative evaluation as an opportunity to screen for OSA, the value of preoperative screening for OSA as a means of decreasing postoperative pulmonary complications has not been established.
Pulmonary Vascular Diseases
The risk of postoperative pulmonary complications in patients with underlying pulmonary vascular disease and intact respiratory mechanics is not known. However, one might anticipate an exaggeration of, or prolongation in, the hypoxemia seen postoperatively (see discussion of gas exchange, above). In addition, pulmonary reserve in these patients is usually reduced; hence, additional pulmonary insults are less likely to be tolerated.
 SMOKING HISTORY
SMOKING HISTORY
That smoking increases the risk of postoperative respiratory complications, even among those without COPD, has long been recognized.44,76 Given the well-documented adverse effects of smoking on respiratory epithelium and pulmonary function, the magnitudes of which correlate with the degree of tobacco consumption, such an association is not surprising.
In individuals undergoing coronary artery bypass graft surgery, the risk of smoking becomes significant when tobacco use exceeds 20 pack-years.77 A statistically significant reduction in complications occurs only when patients discontinue smoking for at least 8 weeks prior to surgery. This finding is consistent with studies showing that abnormalities in pulmonary function may persist up to several months after smoking cessation.78,79 Although smoking cessation of more than 2 months is associated with a decreased risk of postoperative respiratory complications, early retrospective studies actually showed a paradoxical increase in pulmonary complications in patients who stopped smoking only a few weeks or days prior to surgery.80 However, more recent studies81 and analyses82 have failed to demonstrate increased pulmonary complications in patients who quit smoking within 2 months of surgery.
 GENERAL STATE OF HEALTH
GENERAL STATE OF HEALTH
Overall clinical status, as categorized by the American Society of Anesthesiologists’ (ASA) classification (Table 103-7), correlates with development of postoperative pulmonary complications.50,83–85 Specifically, an ASA classification of II or higher is a powerful predictor of increased risk for respiratory problems after surgery.23
TABLE 103-7 American Society of Anesthesiologists’ (ASA) Clinical Classification

 AGE
AGE
Although increased age was previously thought to be a minor risk factor for postoperative respiratory complications,86 more recent analyses indicate that increasing age is, in fact, a significant independent predictor, even after accounting for comorbid conditions associated with aging.23 Advanced age, however, should not be the sole reason for withholding surgery, particularly lung resection.
In one important study, despite the finding of a higher 30-day postoperative mortality in patients over age 70 years who underwent resectional lung surgery, the incidence of postoperative pulmonary complications and hospital stay were not increased, and survival was not decreased, in the older group.87 More recently, in a nested, case-control study of patients greater than age 70 years who underwent lung resection, no significant differences were found between the elderly and younger controls in length of stay, major morbidity, or operative mortality.88 These findings, along with other published results,89 indicate that treatment decisions should be individualized for each patient.
 OBESITY
OBESITY
A number of changes in respiratory mechanics and pulmonary function occur with obesity.90–93 The accumulation of fat in the chest wall, diaphragm, and abdomen may reduce total respiratory compliance by more than 60%—a change that is amplified when the patient assumes the supine position.94 The reduced compliance, in turn, increases the work of breathing.90,91 Consequently, minute ventilation, oxygen consumption, and carbon dioxide production are further increased beyond baseline values, which are already elevated as a result of increased metabolic demands imposed by the obese state. In terms of gas exchange, modest effects at rest are observed only in morbidly obese individuals: lower than predicted PaO2, higher than predicted alveolar-to-arterial oxygen pressure difference, normal blood oxygen saturation, and normal PaCO2.95
Typically, spirometry in obese patients does not indicate airway obstruction. However, a reduction in ERV is found consistently. The magnitude of the reduction correlates with the degree of obesity. Areas of low ventilation relative to perfusion and atelectasis are seen (see discussion of lung volumes, above). In addition to these changes, obese patients appear to have a larger gastric volume and lower gastric pH than do nonobese patients and may be predisposed to aspiration.96
Morbidly obese patients may be at an increased risk for development of atelectasis, as noted on chest radiographs.97,98 However, when bacterial pneumonia, acute respiratory failure, or prolonged mechanical ventilation are considered, and confounding factors are excluded, obese patients undergoing abdominal surgery do not show an increased incidence of clinically significant postoperative pulmonary complications compared with nonobese patients.37,99–101 Major respiratory complications occur in only 4% to 7% of morbidly obese patients undergoing gastric bypass surgery; therefore, in the absence of concurrent cardiopulmonary disease, the risk of postoperative pulmonary complications associated with obesity appears not to be excessive.102 However, as noted previously, obesity is clearly a risk factor for OSA syndrome, which may be unmasked or exacerbated because of use of postoperative analgesics or narcotics.103
 NUTRITIONAL STATUS
NUTRITIONAL STATUS
The effects of malnutrition and severe starvation on the respiratory system include a reduced ventilatory response to hypoxia, decreased diaphragmatic muscle function, impaired cell-mediated and humoral immunity, and alterations in the elastic properties of the lung. Depending on the clinical metrics examined (e.g., weight loss >10% over the previous 6 months, BMI <20 kg/m2), the screening procedures utilized (e.g., Mini Nutritional Assessment (MNA) score <17), and the laboratory tests employed (e.g., serum albumin <36 g/L), malnutrition may be found in 50% or more of hospitalized patients.104,105 Evidence exists that these levels of malnutrition are associated with expiratory muscle weakness (despite preservation of pulmonary function)106 and an increased incidence of postoperative respiratory complications.38,99,107,108 However, aggressive preoperative nutritional support has not been shown to decrease postsurgical pulmonary morbidity.109
 ANTECEDENT RESPIRATORY TRACT INFECTION
ANTECEDENT RESPIRATORY TRACT INFECTION
The effects of an antecedent respiratory tract infection on the incidence of respiratory complications following surgery are difficult to predict. With respiratory syncytial virus (RSV) infections, symptoms typically resolve in 7 to 10 days; viral shedding averages 10 to 13 days, but it may persist beyond 20 days.110,111 Furthermore, enhanced airway reactivity and increased airway resistance associated with this and other viral infections may persist for weeks beyond resolution of acute symptoms.112,113 In addition, diaphragmatic function may be impaired during viral infections.114
In pediatric patients, perioperative, nonlife-threatening, adverse respiratory events are more common in patients with recent (within 4 weeks) upper respiratory tract infections compared with those without infections.115,116 In an adult population, history of an acute respiratory infection in the month preceding surgery is an independent risk factor for development of a postoperative pulmonary complication.41 Therefore, in the setting of an active or recent respiratory tract infection (in the previous 2 weeks), a 2- to 4-week delay in elective surgery is generally advised.
 OTHER PATIENT-RELATED FACTORS
OTHER PATIENT-RELATED FACTORS
Other factors have been reported to be associated with an increased incidence of postoperative pulmonary complications.23 These include congestive heart failure,41,117,118 preoperative functional dependence, impaired sensorium (acute confusion or delirium), and active alcohol use (>2 drinks per day in the past 2 weeks).38,39
INTRAOPERATIVE RISK FACTORS
Several operative factors have been associated with development of pulmonary complications after surgery. These include whether the surgery is emergent or nonemergent, type of anesthesia used, length of the procedure (as determined by the duration of anesthesia), surgical site, and type of surgical incision.
 EMERGENT NATURE OF THE PROCEDURE
EMERGENT NATURE OF THE PROCEDURE
Postoperative pulmonary complication rates are higher for surgeries done on an emergent basis.38,39,41,47,48,117 The increased risk of emergency surgery may be significant23 and may be related to loss of the ability to implement some of the preventative measures outlined subsequently or to deliberately plan the anesthetic strategy.
 TYPE OF ANESTHESIA
TYPE OF ANESTHESIA
The pulmonary effects of general anesthesia have been implicated in the development of postoperative pulmonary complications.38,39,50,51 They include impairment of oxygenation and carbon dioxide elimination. These effects result from anesthetic-induced changes in the shape and motion of the chest wall and diaphragm, which, in turn, lead to increases in alveolar dead space, shunt fraction, and ventilation–perfusion mismatching. Alterations in lung function may contribute to pulmonary morbidity.
Because of the effects of general anesthesia on gas exchange, regional anesthesia (spinal or epidural anesthesia) or local anesthesia with monitored anesthesia care (MAC) has been used as alternatives, particularly in patients with underlying pulmonary disease. Indeed, epidural anesthesia to a T4 sensory level does not appear to alter FRC, VC, FEV1, the alveolar–arterial oxygen gradient, shunt fraction, or cardiac output.119 Overall, regional anesthesia, as compared to general anesthesia, decreases the risk of postoperative respiratory complications, particularly deep vein thrombosis.120,121 These beneficial effects, however, appear to be most significant for abdominal and thoracic procedures122,123 and less so for nonthoracoabdominal procedures, such as carotid endarterectomy124,125 and hip fracture surgery.126,127
 DURATION OF ANESTHESIA
DURATION OF ANESTHESIA
The incidence of pulmonary complications increases significantly for procedures lasting longer than 2 to 4 hours.41,44,45,69,84 Patients whose procedures last 4 hours or more are five times more likely to experience postoperative pneumonia than those whose procedures last less than 2 hours.99
 SURGICAL SITE
SURGICAL SITE
Development of postoperative pulmonary complications has long been recognized to correlate strongly with the anatomic site of operation.41,44,45,69,84 The complication rate (excluding thromboembolic disease) is less than 5% for gynecologic and urologic procedures, 5% to 10% for lower abdominal and head and neck surgeries, and 10% to 20% for upper abdominal surgeries. Abdominal aortic surgery is associated with a pulmonary complication rate of 25% or more.23
Reported postoperative complication rates for thoracic surgery involving lung resection vary from under 10% to 40% or higher.128–133 Postoperative respiratory morbidity following lung resection surgery (in addition to all the factors cited earlier) also depends on a number of other issues, including (1) the presence of underlying lung disease, (2) the amount of functional lung removed, and (3) the extent to which the “bellows” function of the lung is impaired (see discussion of evaluation for lung resection below). Furthermore, the potentially higher incidence of postoperative respiratory complications after lung resection surgery relative to other procedures also reflects the occurrence of specific problems related to entering the pleural space or resection of lung tissue (e.g., development of pleural effusion, empyema, pneumothorax, or persistent pulmonary parenchymal air leak).
 TYPE OF SURGICAL INCISION
TYPE OF SURGICAL INCISION
For abdominal procedures, vertical laparotomy incisions may carry a higher incidence of postoperative complications than do horizontal incisions,134 although this finding has been questioned.135 Abdominal laparoscopic procedures and thoracoscopic lung resection have gained widespread acceptance because of reduced patient discomfort, shortened length of hospitalization, and faster patient return to full activity. Since the magnitude of incisional pain is usually less, and since patients typically ambulate sooner, the incidence of postoperative respiratory complications with these less invasive procedures is likely to be lower. Laparoscopic cholecystectomy, when compared with open cholecystectomy, demonstrates better preservation and faster recovery of lung volumes, higher arterial oxygen saturations, less postoperative pain and analgesia use, and a lower incidence of postoperative pulmonary complications.136–138 Comparable findings have been noted for minimally invasive esophagectomies compared with open procedures, and for video-assisted thoracic surgery versus open thoracotomy.29
POSTOPERATIVE RISK FACTORS
Inadequate pain control, prolonged bed rest, and patient inactivity contribute to development of postoperative respiratory complications.
 INADEQUATE POSTOPERATIVE ANALGESIA
INADEQUATE POSTOPERATIVE ANALGESIA
Effective pain control is vital in the early postoperative period, since pain inhibits coughing and deep breathing and discourages early mobilization—factors that contribute to an increased risk of pulmonary complications. More importantly, there is evidence that effective analgesia after thoracic or abdominal surgery reduces the occurrences of postoperative pulmonary complications.139–142 Obstacles to good postoperative analgesia include hesitancy of the patient to report pain for fear of being labeled a “bad” patient and anxiety of caregivers in administering narcotics because of side effects.
 IMMOBILIZATION
IMMOBILIZATION
Prolonged bed rest and inactivity following surgery impact the risk of postoperative respiratory complications in several ways. FRC decreases by 500 to 1000 mL in moving from the upright to the supine position, favoring development of atelectasis.18 Increased ambulation is associated with better clearance of respiratory secretions. Postoperative immobilization is a major risk factor for development of deep venous thrombosis and pulmonary embolism.
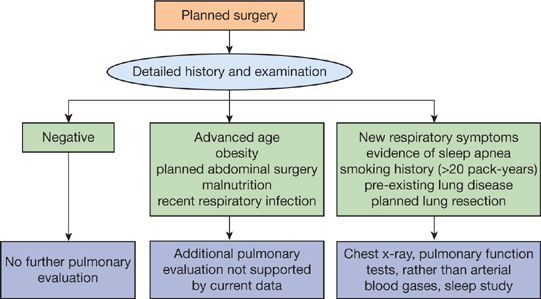
PREOPERATIVE EVALUATION
The principal elements in preoperative evaluation of the surgical patient are (1) the history and physical examination, (2) the chest radiograph, (3) arterial blood gas analysis, and (4) pulmonary function tests (Fig. 103-2).
Figure 103-2 Algorithm for preoperative pulmonary evaluation. See text for discussion.
 HISTORY AND PHYSICAL EXAMINATION
HISTORY AND PHYSICAL EXAMINATION
A careful history is an essential component of preoperative evaluation. The following issues should be reviewed: (1) smoking history; (2) history of respiratory symptoms (e.g., cough, chest pain, dyspnea), including symptoms of sleep apnea; (3) extent of pre-existing lung disease; and (4) history of recent respiratory tract infection. The physical examination is rarely helpful in identifying pulmonary risk factors. When the history is negative, the physical examination is typically unremarkable. However, the initial physical examination supplements the history and provides a baseline for future comparisons.
 CHEST RADIOGRAPH
CHEST RADIOGRAPH
The preoperative chest radiograph is usually unrevealing if risk factors and abnormal physical findings are absent.143,144 Although the admission or screening chest radiograph is more likely to show an abnormality in individuals with known cardiopulmonary disease, the study usually simply confirms the presence of previously known abnormalities; only occasionally does it result in an alteration in management. Thus, a preoperative chest radiograph is indicated when there are new or unexplained symptoms or signs, when there is a history of underlying lung disease and no recent chest radiograph, or when thoracic surgery is planned.
 ARTERIAL BLOOD GAS ANALYSIS
ARTERIAL BLOOD GAS ANALYSIS
Since an elevated PaCO2 is associated with an increased incidence of postoperative respiratory morbidity in patients with significant chronic lung disease, an arterial blood gas analysis should be done preoperatively in these patients.54 Although supportive data are lacking, it is common practice to obtain an arterial blood gas sample in all patients undergoing lung resection surgery, even those without significant underlying lung disease. The determination serves as a basis for comparison with subsequent intra- and postoperative measurements. It is also recommended that an arterial blood gas specimen be obtained in patients who, by either history or physical examination, have a new significant pulmonary process. Data do not support the use of arterial blood gas analysis as a routine preoperative screening test.145
 PULMONARY FUNCTION TESTS
PULMONARY FUNCTION TESTS
An increased risk of postoperative respiratory complications has been demonstrated only with obstructive pulmonary disorders. Although theoretical reasons may prompt expectation of a higher incidence of postoperative respiratory problems in patients with restrictive lung diseases (see discussion of preoperative risk factors, above), currently, data demonstrating a correlation between the degree of restriction (as assessed by lung volumes) and postoperative pulmonary morbidity are lacking. Hence, although a complete battery of pulmonary function tests is useful in evaluating suspected restrictive lung disease, spirometry to evaluate for airway obstruction is all that is required to screen patients at risk for postoperative pulmonary complications.
Indications for preoperative pulmonary function testing include the presence of cough or unexplained dyspnea, a history of chronic lung disease, a history of cigarette smoking (>20 pack-years), or planned lung resection (see below). Current data do not support the routine use of these studies to evaluate the pulmonary risks of advanced age, obesity, malnutrition, or abdominal surgery.146–149 Finally, normal pulmonary function tests obviously do not guarantee a complication-free postoperative course and do not lessen the need for diligent respiratory care following surgery.
EVALUATION FOR LUNG RESECTION
In evaluating patients for lung resection, the clinician must consider two broad issues: (1) What is the surgical morbidity and mortality for the patient with significant underlying chronic lung disease? (2) Will postoperative lung function be adequate to support a reasonable quality of life? Pulmonary function testing, supplemented by exercise testing in selected cases, provides the basis for answering these questions.150,151
 PULMONARY FUNCTION TESTING
PULMONARY FUNCTION TESTING
Studies dating back to the 1950s have shown that the risk of postoperative respiratory complications following pneumonectomy increases significantly when the FEV1 is less than 2 L or 80% of predicted normal, or when the maximal voluntary ventilation (MVV) is less than 50% of predicted.152 For a lobectomy, an FEV1 of 1.5 L appears to be the critical threshold. The diffusion capacity for carbon monoxide (DLCO) has also been identified as a predictor of postoperative complications.153,154 Increased risk is associated with a DLCO of less than 60% to 80% of predicted and appears to be independent from FEV1 as a predictor of complications, morbidity, and death.155–157 Therefore, predictive postoperative lung function should be estimated for patients with an FEV1 or DLCO less than 80% of predicted.150,151
 PREDICTION OF POSTOPERATIVE FEV1 OR DLco
PREDICTION OF POSTOPERATIVE FEV1 OR DLco
Ventilation–perfusion lung scans measure the relative blood flow or ventilation to one lung or lung region and can be used to predict postoperative FEV1 or DLCO using the following equation:

An alternative approach to estimating the predicted postoperative pulmonary function involves a calculation based on the number segments of the lung (10 on the right and nine on the left)158 as follows:
Studies comparing the radionuclide imaging-based technique versus the segment-based method suggest that perfusion imaging, overall, provides a better prediction of postoperative lung function, as the segment method underestimates lung function after pneumonectomy.159,160
In addition to the two methods noted earlier, other approaches have been employed to predict postresection lung function. However, procedures such as quantitative computed tomographic imaging,161 have failed to gain wide acceptance, and oxygen-enhanced MRI,162 although promising, awaits further development.
A number of studies have demonstrated that the perioperative risk for lung resection increases significantly when the predicted postoperative FEV1 or DLCO has <40% of predicted.153,163–166 Therefore, a predicted postoperative FEV1 or DLCO ≥40% predicted has been proposed as useful criteria for undertaking “safe” pulmonary resection.150,167 Finally, with recognition that most patients who undergo resectional lung surgery have lung cancer, and that this malignancy has virtually a 100% mortality without surgery (for nonsmall cell tumors), caution must be exercised in applying exclusionary criteria for surgery.
 EXERCISE TESTING
EXERCISE TESTING
A number of investigators have found measurement of maximal oxygen consumption (VO2max) during cardiopulmonary exercise testing to be useful in predicting postoperative morbidity and mortality.168–172 Specifically, a ![]() of less than 15 to 20 mL/kg/min is associated with an increased incidence of postoperative complications. Cardiopulmonary exercise testing is, therefore, used to further assess the operability of patients of who would be at high risk for surgery based on determination of predicted postoperative pulmonary function.150
of less than 15 to 20 mL/kg/min is associated with an increased incidence of postoperative complications. Cardiopulmonary exercise testing is, therefore, used to further assess the operability of patients of who would be at high risk for surgery based on determination of predicted postoperative pulmonary function.150
If cardiopulmonary exercise testing is unavailable, two technologically simpler approaches, stair climbing and a walking test, can be used to assess a patient’s fitness for lung resection. Although standardization of the stair climbing test may be problematic, demonstration of a patient’s ability to climb five flights of stairs predicts a ![]() >20 mL/kg/min; patients who are unable to climb one flight of stairs have a
>20 mL/kg/min; patients who are unable to climb one flight of stairs have a ![]() <10 mL/kg/min.173 Furthermore, an ability to climb three flights of stairs reliably identifies patients who are likely to do well after a lobectomy, despite having a predicted postoperative FEV1 or DLCO that is <40% of predicted.174,175
<10 mL/kg/min.173 Furthermore, an ability to climb three flights of stairs reliably identifies patients who are likely to do well after a lobectomy, despite having a predicted postoperative FEV1 or DLCO that is <40% of predicted.174,175
The shuttle walk and 6-minute walk tests have also been investigated as alternatives to cardiopulmonary exercise testing. For the shuttle walk, the patient walks back and forth over a distance of 25 m at a progressively faster rate. Inability to complete 25 shuttles approximates a ![]() <10 mL/kg/min.176,177 A 6-minute walk distance >1000 ft has been reported as predictive of successful surgical outcome.164
<10 mL/kg/min.176,177 A 6-minute walk distance >1000 ft has been reported as predictive of successful surgical outcome.164
 RECOMMENDED APPROACH
RECOMMENDED APPROACH
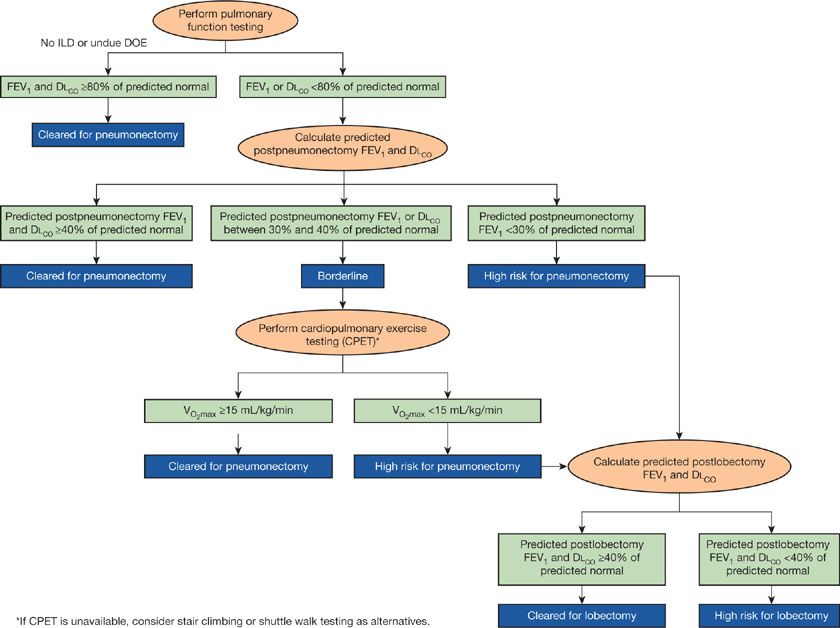
Summarized in Fig. 103-3 is a recommended sequence for evaluating patients for lung resection. The approach is adapted from recommendations of the American College of Chest Physicians (ACCP),150 which, despite some differences, is also consistent with guidelines from several European professional societies.151,167 The approach is predicated on determining the patient’s operability for pneumonectomy, should this procedure be deemed necessary at the time of surgery (e.g., to permit complete removal of a tumor or to deal with an unanticipated intraoperative complication):
• If the preoperative FEV1 and the DLCO are ≥80% predicted normal and the patient has no dyspnea on exertion or interstitial lung disease, the patient is cleared for pneumonectomy; no further testing is required.
• If the preoperative FEV1 or the DLCO are <80% predicted normal, the predicted postpneumonectomy FEV1 and DLCO should be estimated.
• If the predicted postpneumonectomy FEV1 and DLCO are ≥40% of predicted normal, the patient is cleared for pneumonectomy and no further testing is required.
• If the predicted postpneumonectomy FEV1 is <30% predicted normal, the patient is considered at high risk for a pneumonectomy.
• If the patient is considered at borderline risk for pneumonectomy (the predicted postoperative FEV1 or DLCO is <40% but ≥30 predicted normal), exercise testing should be done. Borderline patients with a ![]() ≥15 mL/kg/min are cleared for pneumonectomy (no further testing is required), while patients with
≥15 mL/kg/min are cleared for pneumonectomy (no further testing is required), while patients with ![]() <15 mL/kg/min are considered at high risk for a pneumonectomy.
<15 mL/kg/min are considered at high risk for a pneumonectomy.
• If the patient is determined to be at high risk for pneumonectomy, the predicted postlobectomy FEV1 and DLCO should be determined to inform conversations about the risks and benefits of more limited surgical resections or nonoperative treatment options.
Figure 103-3 Algorithm for preoperative evaluation for lung resection. See text for discussion. ILD, interstitial lung disease; DOE, dyspnea on exertion.
For selected, high-risk patients with emphysema and potentially resectable lung cancer, consideration should be given for a segmental resection178 or combined cancer resection and lung volume reduction.179 The latter is particularly worth considering if the emphysema is heterogeneous and primarily involves the lobe to be resected.
PREOPERATIVE PREPARATION
Relatively few studies have specifically addressed the question of whether aggressive, preoperative pulmonary preparation decreases postoperative pulmonary morbidity and mortality.180 Although data on preoperative preparation are limited, for patients with active obstructive airway disease, intensive preoperative respiratory therapy (bronchodilators, corticosteroids, antibiotics, and chest physiotherapy) does appear to reduce the incidence of postoperative respiratory complications by more than 50%.84,181–183
Several preoperative prophylactic measures should be considered in patients undergoing elective surgery (Table 103-8).