■ Once the history, physical examination, and noninvasive testing are complete, the surgeon must determine the next step in imaging, which may be both diagnostic and therapeutic. The patient’s functional status, cardiac risk profile, and other comorbidities (ambulatory status, etc.) will all play an important role in determining whether any revascularization attempt is even feasible. If femoral pulses are not palpable on physical examination and noninvasive testing is also suggestive of inflow disease, computed tomography arteriography (CTA) may be instrumental in evaluating the extent of aortoiliac disease, taking into account renal function and risk of contrast-induced nephrotoxicity. Alternatively, aortography obtained from contralateral femoral access or upper extremity arterial access may suffice. General management approaches to aortoiliac versus infrainguinal disease are discussed elsewhere in this book.
■ Once the clinical determination is made that the level of disease is infrainguinal, digital subtraction angiography is an excellent diagnostic test and provides access for therapeutic intervention as well. This chapter focuses on patients with multilevel infrainguinal disease who, presumably, have either failed endovascular revascularization, prior open bypass, or who have no endovascular options for revascularization. Therefore, the primary goal of diagnostic angiography is to assess the caliber and quality of inflow vessels and bypass targets.
■ The contralateral femoral artery is accessed and an aortogram with oblique pelvic views is obtained to rule out the presence of inflow disease that may need treatment prior to evaluating the infrainguinal segment. An ipsilateral oblique magnified projection will be helpful in determining if there is any significant common femoral or profunda femoris stenosis, which is especially important if these vessels are to be chosen as the source of inflow for an open bypass (FIG 2).
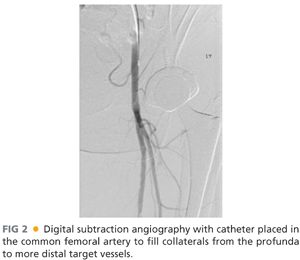
■ Deciding on which type of intervention to perform, endovascular versus open, and how aggressive to be about either approach is determined by multiple considerations as outlined earlier and elsewhere in this section. Patients requiring open bypass typically have multilevel disease. As such, it is critically important to image the runoff with the catheter placed proximal to the profunda origin, as the distal runoff will likely be filling from profunda collaterals that are communicating with geniculate collaterals. With common femoral or profunda disease, the catheter will need to be placed in the common iliac artery to evaluate internal iliac collaterals that are in communication with the profunda and more distal collaterals. Performing magnified, time-delayed digital subtraction angiography will assist in revealing which tibial vessels are patent and filling through collateral networks. It is also essential to identify the primary named crural artery that is in continuity to the foot that will perfuse the tissue affected by ischemic ulceration (FIG 3).
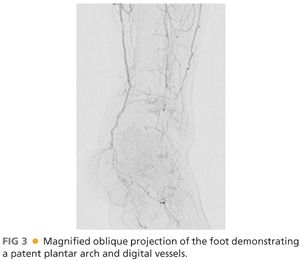
■ Using full-strength contrast, magnified projections of the foot will help delineate which pedal vessels are patent, which fill the tarsal and plantar branches, and confirm the status of the plantar arch. Occasionally, there are situations in which no suitable target (e.g., “named” artery) is identifiable and exploration of a tibial vessel at the time of operative intervention may be required. This exercise is fraught with risk, however, especially in the setting of a desperately ischemic foot, and should rarely be undertaken without conclusive pre- or intraoperative arteriography. Duplex ultrasonography may assist in further defining quality, caliber, and patency of tibial vessels in these situations. Choosing a patent posterior tibialis or anterior tibialis artery in direct continuity with the pedal arteries is preferred over a peroneal artery as a distal target when the former is available, especially in cases of forefoot wounds; however, peroneal arteries are perfectly suitable and serviceable in this situation in the absence of other alternatives.
■ The decision to proceed with open perimalleolar bypass is made in the context of the patient’s overall clinical functional status, cardiopulmonary and renal comorbidities, presence of autogenous saphenous vein conduit, and options for endovascular revascularization. Preoperative autogenous conduit assessment is best performed by detailed ultrasonographic imaging along the length of the vein. Preference is always given to a single segment of greater saphenous vein (GSV) from the ipsilateral leg that is at least 2.5–3 mm in diameter, compressible, and free of thrombus throughout. Assessment of the contralateral GSV is useful in case ipsilateral vein is found to be of poor quality during operative exploration.
■ Inflow artery selection is usually based on length of available conduit and location of proximal disease. The common, superficial, or deep femoral or popliteal arteries may all serve as suitable inflow arteries in circumstances where minimal or insignificant occlusive disease is present proximally. This determination is best made during diagnostic angiography. The need for concomitant inflow endarterectomy should also be evaluated at this time.
SURGICAL MANAGEMENT
Preoperative Planning
■ Type of anesthesia to be used is determined by the type of cardiopulmonary comorbidities and the anatomic level of arterial occlusive disease. Preoperative consultation with anesthesiology and cardiology is customary in this patient population to assess the appropriate amount of surgical risk. General anesthesia, peripheral nerve block, and spinal anesthesia are all potential options in this group of patients. Intraoperative fluid administration should be used judiciously and preoperative preparation should include blood type determination and crossmatching as necessary. It is our practice to hold therapeutic anticoagulation for at least a few days prior to the procedure, but most patients remain on antiplatelet agents through the day of surgery and continue through the perioperative period.
Positioning
■ Any lower extremity bypass might require intraoperative angiography and, as such, all such procedures should be performed on a radiolucent table. The patient is positioned supine, with the leg slightly abducted and externally rotated to provide optimal exposure of the ipsilateral GSV harvest site (FIG 4). It is our practice to localize the GSV by ultrasound to assist in incision planning. This also helps determine whether the contralateral leg should also be prepped as an alternative site for vein harvesting.
■ Other items that should be available in the room include a sterile pneumatic tourniquet and surgical bump. Both are useful when the below knee popliteal artery or tibioperoneal trunk is used for arterial inflow for the graft. Open forefoot wounds are excluded from the operative field with adhesive or Ioban drapes.
TECHNIQUES
PERIMALLEOLAR BYPASS TO THE DISTAL POSTERIOR TIBIALIS ARTERY
First Step: Exposure of the Posterior Tibialis Artery at the Ankle
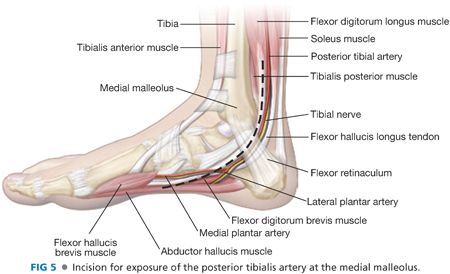
■ Simultaneous dissection of the inflow and outflow targets increases the efficiency of the operative approach. The distal incision is marked by palpating posterior to the medial malleolus, taking care to avoid injury to adjacent GSV (FIG 5). Dissection is carried sharply through skin and subcutaneous tissues and through the flexor retinaculum. The tendons of the flexor digitorum longus muscle and flexor hallucis longus pass anteriorly and posteriorly, respectively, to the neurovascular bundle at this level. The paired tibial veins are often seen first as overlying the artery. The tibial nerve travels posterior to the artery and may not be seen clearly during this exposure. The tibial artery does not need to be dissected circumferentially if a pneumatic tourniquet is deployed for proximal control. Use of a tourniquet minimizes risk for venous injury at the dissection site and trauma to the arterial endothelium from vessel loops and vascular clamps. For exposure of the medial and lateral plantar arteries, the same incision is typically carried further distally onto the medial aspect of the foot.
Second Step: Exposure of the Inflow Artery
■ Concurrent dissection of the arterial inflow should be performed while the tibial target is being exposed. If the common femoral artery is chosen, then a longitudinal vertical incision just below the inguinal ligament will allow for simultaneous exposure of the femoral bifurcation in addition to the saphenofemoral junction for dissection of the GSV (FIG 6). If the deep femoral artery is to be used as inflow, then division of the lateral femoral circumflex vein may be helpful in controlling the first-order branches past the origin. If the deep femoral origin is truly deep, the muscle bellies of the adductor longus and vastus medialis should be divided to limit the angle from which the graft originates from the arterial anastomosis. If the below knee popliteal artery is to be used as the inflow, which might be the case in diabetic patients with severe tibial disease not amenable to endovascular revascularization, this exposure is best obtained through a medial calf incision.
Third Step: Harvest and Preparation of Autogenous Vein
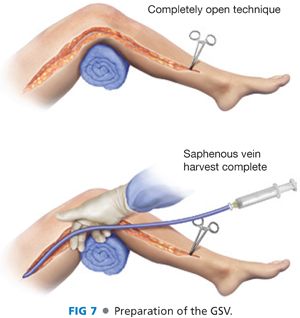
■ The course of the GSV is marked on the skin prior to prepping. The shortest segment of suitable caliber and quality GSV is harvested. Incision placement is partially determined by the location of the arterial access incisions. Care should be taken to avoid creating skin flaps during vein exposure. Harvesting vein through skip incisions may help to minimize wound complications but is not necessary for a good result. Minimal vein manipulation, with care being taken to accurately ligate side branches with 4-0 braided permanent suture (or silk), is important. Minimal dissection of the vein is typically required when in situ bypass in planned. The vein can be left in its bed, with limited dissection of the anastomotic segments. Skip incisions provide access to ligate large side branches.
■ After an adequate length of saphenous is exposed, it is removed from its bed for bypass placement or transposed into position after valve lysis for in situ bypass. When removed and repositioned, we generally try to use the largest diameter segment for bypass. The saphenofemoral junction is oversewn or suture ligated. The vein is then reversed and distended gently with heparinized saline or plasmalyte solution. Other vein preservative solutions can be used at the discretion of the surgeon. Any untied branches are carefully clamped and tied with silk suture. Small tears or holes are oversewn in a longitudinal fashion, taking care not to narrow the vein, using 7-0 polypropylene suture (FIG 7). Depending on institutional expertise, endovein harvest is an alternative method to minimize incisional length and potential wound complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


