CHAPTER 95 Pericardium and Constrictive Pericarditis
THE PERICARDIUM
History
The earliest descriptions of the pericardium date back to Hippocrates (460 to 377 BC).1 Galen (129 to 210 AD) described the protective function of the pericardium and also reported a pericardial effusion in animals. Avenzoar (1091 to 1162) described pericarditis,2 and Vesalius (1514 to 1564) carefully documented the anatomy of the pericardium. Jean Riolan (1649) suggested treating pericarditis with trephination of the sternum, and a case of hemopericardium was reported by William Harvey (1649). The conditions of cardiac tamponade and constrictive pericarditis were described by Richard Lower (1669), John Mayow (1674), and Morgagni (1756). The pathophysiology of constrictive pericarditis was further clarified by Cheevers in 1842.
Anatomy
The pericardium is a fibrous sac surrounding the heart and mediastinal great vessels. The outer wall of the pericardial sac consists of an outer fibrosa and an inner serosa.3 Histologically, the fibrosa is fibrocollagenous tissue with elastic fibers oriented along the lines of stress, and the pericardial serosa is composed of mesothelial cells with microvilli and an underlying basal lamina.3
This outer pericardial sac folds onto the heart and great vessels, where the epicardium and outer adventitial layer of the heart and great vessels constitute the visceral lining of the pericardial sac. Laterally, the pericardium forms the medial walls of the pleural spaces. Inferiorly, the pericardium is the superior surface of the central tendon of the diaphragm, and, superiorly, the pericardium blends with the deep cervical fascia. Anteriorly, the pericardium is loosely joined to the xiphoid process and the sternal manubrium by ligamentous structures. The anterior pericardium is otherwise separated from the posterior sternum by loose connective tissue and the thymus. Posteriorly and superiorly, the pericardium envelops the great vessels, the venae cavae, and the pulmonary veins. The posterior pericardial space has two developmental recesses: the transverse sinus separating the great vessels from the pulmonary veins and the oblique sinus separating the left and right pulmonary veins (Fig. 95-1).3
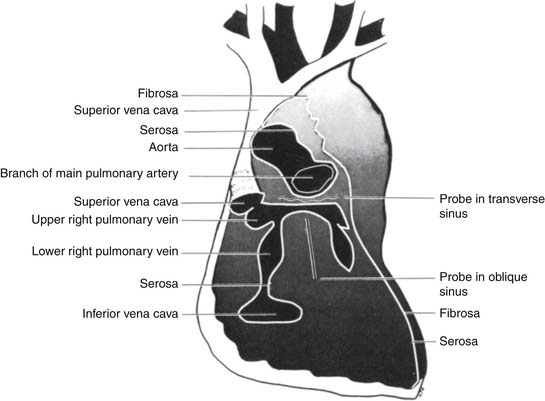
Figure 95–1 Anatomy of the posterior pericardial reflections. Note the transverse sinus and the oblique sinus.
(From Spodick DH. The pericardium. New York: Marcel Dekker; 1997.)
The arterial blood supply and venous drainage of the pericardium come from the pericardiophrenic branches of the internal mammary vessels bilaterally. The lymphatic drainage of the visceral pericardium is the tracheal and bronchial lymph chain, and the parietal pericardium shares lymphatic drainage with the sternum, diaphragm, and mid mediastinum. The pericardium is innervated from the phrenic nerves, with some vagal innervation via the esophageal plexus.3
The pericardium normally contains 15 to 35 mL of serous fluid. Pericardial fluid is a transudate containing less protein but more albumin than serum. Pericardial fluid therefore has a lower osmolality than does plasma.3
Normal Physiology
At rest, the normal pericardium probably has little restraining effect on cardiac systolic or diastolic function.4 However, under conditions of acute cardiac dilation, the normal pericardium probably does increase diastolic stiffness and limit diastolic filling of both left and right ventricles.5–7 Under normal conditions, relatively little interaction between the left and right ventricles is mediated by the pericardium.8,9
Pathophysiology
Pericardial Effusion
Pericardial effusion (more than 50 to 100 mL) is the simple result of more fluid transuding into the pericardial space than is resorbed. Pericardial effusions requiring drainage typically contain 500 to 700 mL of fluid.10 Eventually, pericardial pressure rises high enough that resorption matches fluid production. Sustained increases in pericardial pressure over time cause the pericardial sac to stretch (as a result of material plasticity, or creep) with slippage of pericardial collagen fibers and pericardial thinning. Causes of increased pericardial fluid production include inflammation or infection of the pericardium. Decreased pericardial fluid resorption can result from venous hypertension or lymphatic obstruction. If pericardial effusion increases pericardial pressure sufficiently, cardiac tamponade and pulsus paradoxus can result (see later).
Cardiac Tamponade
Cardiac tamponade has been defined as hemodynamically significant cardiac compression from accumulating pericardial contents that evoke and defeat compensatory mechanisms.3 Cardiac tamponade can result from pericardial fluid, pus, blood, air, or tumor. In a normal pericardium, roughly 200 mL of acute pericardial fluid accumulation can produce tamponade, but larger volumes may be required in chronically enlarged pericardial sacs.
Diagnostic signs of cardiac tamponade are listed in Box 95-1.
Pulsus Paradoxus
Cardiac tamponade is associated with pulsus paradoxus, defined as a fall in systolic blood pressure of greater than 10 mm Hg with inspiration. Pulsus paradoxus is thus an exaggeration of the normal inspiratory decrease in systolic blood pressure due to the same mechanisms (see Normal Physiology, earlier). Although pulsus paradoxus is characteristic of cardiac tamponade, pulsus paradoxus can also be seen in chronic obstructive pulmonary disease, pulmonary embolism, obesity, right heart failure, and ascites, where it occurs by the same mechanisms.8 Pulsus paradoxus may be absent in cardiac tamponade with severe left ventricular dysfunction, atrial septal defect, severe aortic regurgitation, or positive pressure breathing.3 Proposed mechanisms of pulsus paradoxus include the following:
Constrictive Pericarditis
Constrictive pericarditis results when the volume of the pericardial sac itself is sufficiently reduced relative to cardiac volume that cardiac filling is impaired. In constrictive pericarditis, pericardial fluid is generally absent or of normal volume. The wall of the pericardial sac is almost always thickened in constrictive pericarditis and may be 3 to 20 mm thick, as opposed to the 1- to 2-mm thickness of normal pericardium (Box 95-2).
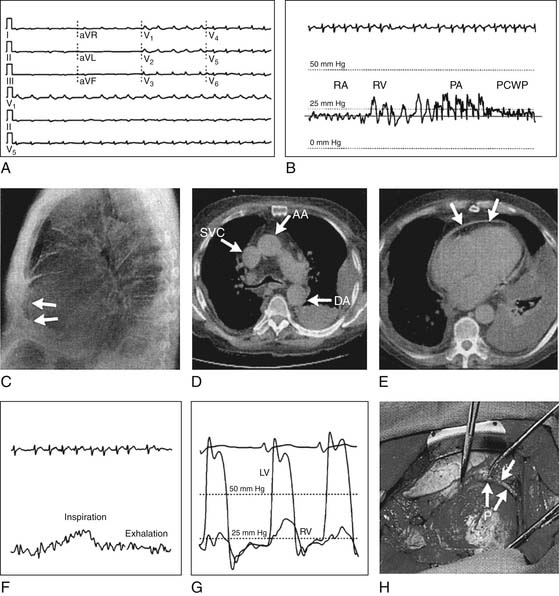
Unlike cardiac tamponade, constrictive pericarditis impairs cardiac filling only in late diastole. Thus, early diastolic filling of the right ventricle occurs briefly in constrictive pericarditis until the ventricle suddenly reaches the rigid constraint of the pericardium. The result is the pathognomonic “square-root” sign in the right and left ventricular diastolic filling pressure waveforms (Fig. 95-2).
Similarly, in constrictive pericarditis, the central venous pressure tracing has a prominent y descent that corresponds to the initial dip of the square-root sign of the right and left ventricular tracings. This y descent normally results from “diastolic collapse” of the normal venous pressure as rapid atrial filling occurs, and the y descent is exaggerated by constrictive pericarditis.
Constrictive pericarditis is associated with inspiratory jugular venous distension (Kussmaul’s sign) (see Fig. 95-2), which is less frequent in cardiac tamponade. Kussmaul’s sign can also occur in right ventricular failure, restrictive cardiomyopathy, cor pulmonale, and acute pulmonary embolism.
Chronic elevation of venous pressures in constrictive pericarditis can result in hepatic congestion, cardiac cirrhosis, protein-losing enteropathy, and nephrotic syndrome. Chronically, constrictive pericarditis alters the neurohormonal axis with elevations of serum norepinephrine, renin, aldosterone, cortisol, growth hormone, and atrial natriuretic peptide.14
The differential diagnosis of constrictive pericarditis consists primarily of restrictive cardiomyopathy which can occur simultaneously with constrictive pericarditis.7,15 Characteristic histology on myocardial biopsy, pulmonary capillary wedge pressure more than 5 mm Hg greater than central venous pressure, slower early diastolic filling, and impaired left ventricular systolic function all favor restrictive cardiomyopathy over constrictive pericarditis. Acute volume loading of 500 mL during right heart catheterization accentuates the right-sided pressure findings of constrictive pericarditis and produces smaller changes in restrictive cardiomyopathy.
Diagnosis of Pericardial Disease
History and Symptoms
The presenting symptoms of pericardial disease can include fever, malaise, chest discomfort, shortness of breath, pedal edema, and abdominal distension. Past medical history may reveal prior chest trauma, chest irradiation, or exposure to infectious agents such as Mycobacterium tuberculosis. The time course of pericardial disease has been termed acute (<3 months), chronic (>3 months), and recurrent.7
Electrocardiogram
Pericardial disease can be associated with atrial fibrillation, and the electrocardiogram (ECG) may show diminished QRS voltage (see Fig. 95-2). The four stages of ECG changes in acute pericarditis are as follows:
Echocardiogram
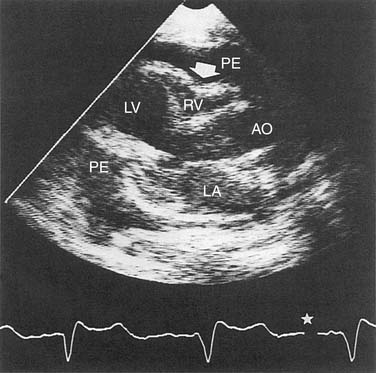
It is easy to detect loculated or generalized pericardial effusion on an echocardiogram. An echo-free pericardial space of less than 10 mm is termed a small effusion, whereas circumferential spaces of 10 to 20 mm and greater than 20 mm are considered moderate and large pericardial effusions, respectively.7 The echocardiogram can also be used to diagnose intrapericardial masses, pericardial cysts, pericardial calcification or thickening, or associated cardiac disease. The echocardiogram can be diagnostic of cardiac tamponade with findings of end-diastolic right atrial collapse, diastolic right ventricular collapse, and wide respiratory variation of blood flow velocity through cardiac valves (Fig. 95-3).16
Computed Tomography
Computed tomography (CT) can demonstrate pericardial effusion, pericardial calcification and thickening, intrapericardial masses, and pericardial cysts (see Fig. 95-2). CT may be more sensitive than magnetic resonance imaging (MRI) in detecting calcification, but it has the disadvantages of requiring intravenous contrast injection and having more motion artifact.17
Magnetic Resonance Imaging
MRI can demonstrate pericardial effusion, pericardial thickening, intrapericardial masses, pericardial cysts, and even intracardiac disease.18 Relative to CT, MRI has the advantages of not requiring intravenous contrast injection and of having less motion artifact. MRI may be less sensitive than CT for detecting pericardial calcification. Newer magnetic imaging techniques such as steady-state free-procession and spin tagging may improve information over older T1- or T2-weighted spin echo techniques.18
Cardiac Catheterization
Cardiac fluoroscopy can demonstrate pericardial calcification or abnormally prominent swinging motion of the left ventricle over the cardiac cycle in pericardial effusion. Right heart catheterization can be diagnostic of cardiac tamponade or pericardial constriction (see earlier) (see Fig. 95-2). Both tamponade and constriction show equalization and elevation of diastolic pressures in all four chambers of the heart. Physiologic tamponade or constriction with right atrial mean pressure less than 14 mm Hg is unusual. Constriction may be differentiated from tamponade or restrictive cardiomyopathy by the square-root sign in the right and left ventricular tracings (see Fig. 95-2), and the findings of cardiac constriction are accentuated by acute 500-mL volume challenge (see Constrictive Pericarditis, earlier).
Pericardiocentesis
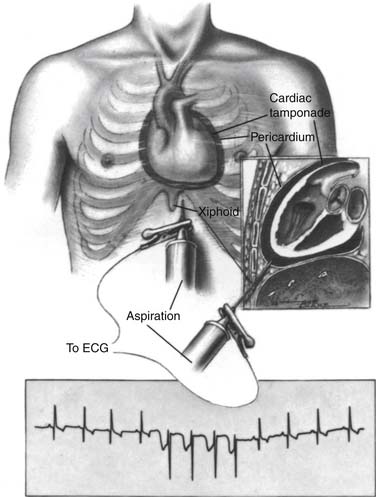
Pericardiocentesis is a means to rapidly treat cardiac tamponade, to prevent tamponade in large effusions, and to obtain pericardial fluid for diagnosis.15 Contraindications include aortic dissection, coagulopathy, small or loculated effusions, traumatic hemopericardium, and nonviral infectious pericarditis. Pericardiocentesis is generally performed with the patient supine and using local anesthesia. A long pericardial needle is inserted into the patient’s pericardial space left of the xiphoid, inferior to the left costal margin (Fig. 95-4). The needle is advanced toward the patient’s left mid scapula while maintaining aspiration pressure on the needle. Cardiac puncture can be avoided by a combination of echocardiography and monitoring an ECG obtained by an electrode attached to the needle. The needle is withdrawn if the ECG shows sudden negative deflection of the QRS complex, indicating contact with the myocardium.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree