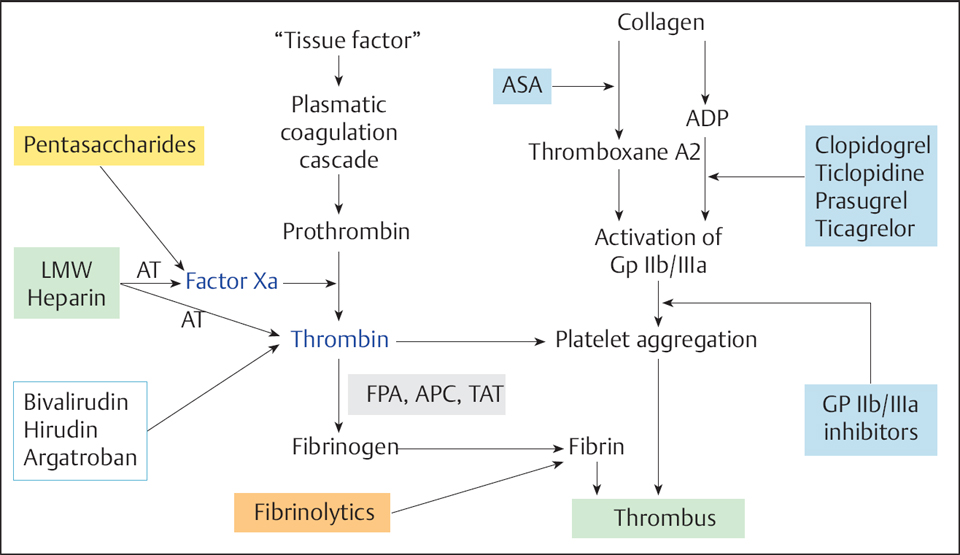
28 Peri- and Postinterventional Antithrombotic Therapy for Coronary Interventions Catheter interventions are associated with trauma to the vessel wall. In addition to the acute changes in flow resulting from a dissection membrane, endothelial injury leads to activation of the coagulation system, with increased platelet aggregation and thrombus formation. In the absence of sufficient antithrombotic therapy, the thrombus formation can lead to abrupt vessel closure during or following percutaneous coronary intervention (PCI). Furthermore, the thrombus is a basis for the migration of smooth muscle cells of the media, a factor in the development of restenosis. Platelets, the coagulation system, and vascular wall structures are synergistically involved in thrombus formation. The local activation of the coagulation system at the injured vessel wall can occur by different mechanisms (Fig. 28.1): These initial physiological processes for the primary hemostasis after a vessel injury can result in a chain reaction with the formation of large platelet thrombi. The platelet thrombus itself further activates the coagulation system and thrombin. Accordingly, antithrombotic therapy after coronary interventions is aimed at In recent years, antithrombotic therapy has been enhanced by the addition of new antithrombotic and platelet-inhibiting compounds (Fig. 28.3). The current approaches will be briefly characterized in the following sections. Then the pharmacological strategies for different clinical settings will be described. Unfractionated heparin is a mixture of sulfated mucopolysaccharides with a wide range of molecular weights (between 3,000 and 50,000 daltons). Heparin is an indirect thrombin inhibitor. The anticoagulant effect depends on the presence of antithrombin III, which forms a complex with heparin. The heparin–antithrombin complex inactivates the coagulation factors XIIa, XIa, Xa, IXa, and IIa (thrombin), where thrombin and Xa are the most important components. Thrombin already bound in a thrombus cannot be inhibited by heparin, which means that residual thrombi retain their procoagulatory effect. Dosage. Heparin doses that have been recommended for PCI range between 5,000 and 15,000 IU. For elective PCI most operators routinely administer 10,000 IU by the IV or IC routes; others adjust the heparin dose to body weight (70–100 IU/kg) or titrate the heparin dose according to the activated clotting time (ACT; 250–350 seconds). If concomitant administration of a GP IIb/IIIa inhibitor is planned, the heparin doses and the target ACT (200–250 seconds) will be lower. There is no clear correlation between ACT and periprocedural adverse event rate; the only well established point is that the rate of bleeding complications increases with an ACT > 400 to 600 seconds. Dose recommendations according to the clinical setting are provided below. Indications. Heparin administration after PCI depends on the clinical setting and is done either as pure thrombosis prophylaxis (with low-molecular-weight heparin, for example) or therapeutically for acute coronary syndrome. For individualized patient management the following observations are of significance. Adverse effects. The major adverse effects of heparin therapy are the bleeding complications with high doses and, with extended administration, heparin-induced thrombocytopenia (HIT). Two types of HIT are differentiated (Table. 28.1): The HIT forms are differentiated clinically and by demonstration of HIT antibodies. Table 28.1 Differentiation of heparin-induced thrombocytopenia type I and type II
 Basics
Basics
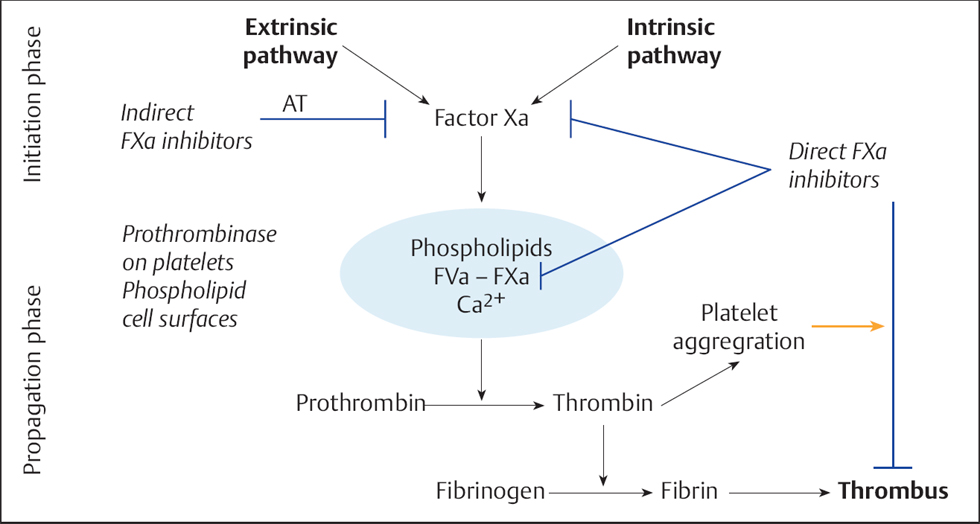
 There is marked activation of the extrinsic coagulation cascade when subendothelial vessel structures are exposed, such as the collagen fibers of the basal membrane, and the release of tissue factor (TF), a membrane-bound protein of the vascular smooth muscle cells and connective tissue cells of the vessel wall. In contrast, locally impaired blood flow due to a dissection membrane or due to an induced lesion activates the intrinsic coagulation cascade. Both pathways lead via activation of the prothrombin complex to an increased formation of thrombin (Fig. 28.2). Thrombin is the most potent platelet activator.
There is marked activation of the extrinsic coagulation cascade when subendothelial vessel structures are exposed, such as the collagen fibers of the basal membrane, and the release of tissue factor (TF), a membrane-bound protein of the vascular smooth muscle cells and connective tissue cells of the vessel wall. In contrast, locally impaired blood flow due to a dissection membrane or due to an induced lesion activates the intrinsic coagulation cascade. Both pathways lead via activation of the prothrombin complex to an increased formation of thrombin (Fig. 28.2). Thrombin is the most potent platelet activator.
 Thrombin inhibition
Thrombin inhibition
 Inhibition of platelet aggregation
Inhibition of platelet aggregation
Unfractionated Heparin
 For elective, uncomplicated PCI no relationship between rate of cardiac complications and heparin dose has been established.
For elective, uncomplicated PCI no relationship between rate of cardiac complications and heparin dose has been established.
 High heparin doses mean an increased risk of vascular complications.
High heparin doses mean an increased risk of vascular complications.
 Prolonged heparin infusions after elective PCI with good procedural success do not offer an advantage regarding cardiac complications, but rather increase the risk of vascular complications.
Prolonged heparin infusions after elective PCI with good procedural success do not offer an advantage regarding cardiac complications, but rather increase the risk of vascular complications.
 For stenoses with increased thrombus burden, especially in patients with acute coronary syndrome and aortocoronary vein grafts, pretreatment with heparin or low-molecular-weight heparin and with antiplatelet therapy prior to PCI lowers the rate of acute complications.
For stenoses with increased thrombus burden, especially in patients with acute coronary syndrome and aortocoronary vein grafts, pretreatment with heparin or low-molecular-weight heparin and with antiplatelet therapy prior to PCI lowers the rate of acute complications.
 HIT type I is characterized by a transient decrease in the platelet count, which typically occurs during the first 10 days of a high-dose intravenous therapy with unfractionated heparin. HIT type I does not lead to thromboembolic complications.
HIT type I is characterized by a transient decrease in the platelet count, which typically occurs during the first 10 days of a high-dose intravenous therapy with unfractionated heparin. HIT type I does not lead to thromboembolic complications.
 With HIT type II a complex develops between heparin and platelet factor 4, against which antibodies are formed. The antigen–antibody complexes adhere to platelets and result in platelet activation, with increased formation of platelet factor 4 and further antibody generation. Despite at times severe thrombocytopenia of < 30,000/μL, bleeding complications are rare. Thromboembolic complications in the arterial and venous circulation predominate. Due to the white platelet thrombi the disease is also called “white clot” syndrome.
With HIT type II a complex develops between heparin and platelet factor 4, against which antibodies are formed. The antigen–antibody complexes adhere to platelets and result in platelet activation, with increased formation of platelet factor 4 and further antibody generation. Despite at times severe thrombocytopenia of < 30,000/μL, bleeding complications are rare. Thromboembolic complications in the arterial and venous circulation predominate. Due to the white platelet thrombi the disease is also called “white clot” syndrome.
 If HIT is clinically suspected but antibodies are negative, heparin should be discontinued and replaced with argatroban or bivalirudin.
If HIT is clinically suspected but antibodies are negative, heparin should be discontinued and replaced with argatroban or bivalirudin.
| HIT type I | HIT type II |
Incidence | 5–30 % of all heparin-treated patients | ~ 0.5 % of all heparin-treated patients |
Heparin | Usually high-dose standard heparin IV | Independent of heparin type, route of administration, and dosing |
Pathogenesis | Direct interaction between heparin and platelets | Immunological reaction |
Platelets | Rarely decrease to ≤ 100,000/μL | Markedly < 100,000/μL |
Timing | During the first 10 days | Between day 5 and day 20 |
Complications | None | Venous/arterial thromboses |
Mortality | None | ~ 20 % |
The disadvantages of unfractionated heparin are
 Variable antithrombotic effect due to strong plasma protein binding
Variable antithrombotic effect due to strong plasma protein binding
 Lack of inhibition of thrombus-bound thrombin
Lack of inhibition of thrombus-bound thrombin
 Heparin-induced platelet activation
Heparin-induced platelet activation
 Can be inhibited by platelet factor 4
Can be inhibited by platelet factor 4
Low-molecular-weight Heparins
Whereas unfractionated heparin inhibits factor Xa and thrombin to a similar degree, low-molecular-weight heparins inhibit predominantly factor Xa and therefore have advantages from a pharmacological standpoint. Low-molecular-weight heparins, which are manufactured by fractionation of standard heparin, are also heterogeneous, but their range of molecular weights is markedly reduced, usually 4,000 to 9,000 daltons. This range is predominantly active against Xa. Higher-molecular-weight heparins, which inhibit thrombin, are absent.
While low-molecular-weight heparin was initially used predominantly for thrombosis prophylaxis, itis now an established component in the treatment of unstable angina in combination with antiplatelet therapy. The extent of anticoagulation cannot be assessed either by ACT or by aPTT, so that the peri-interventional administration of low-molecular-weight heparin for elective patients is not standard according to current guidelines. Nevertheless, there are several ongoing studies that test the safety of standard doses in combination with antiplatelet therapy.
The clearance of low-molecular-weight heparins is slower than that of unfractionated heparin, so that a daily subcutaneous injection is sufficient for thrombosis prophylaxis. For therapeutic anticoagulation, variable weight-adjusted doses up to twice daily are required, depending on the substance used. For peri-interventional anticoagulation, data are available essentially only for enoxaparin.
Direct Thrombin Inhibitors
In contrast to heparins, direct thrombin inhibitors also bind thrombus-bound thrombin and thus prevent thrombin-induced platelet aggregation. The clinical results with the direct thrombin inhibitors hirudin and bivalirudin are in part inconsistent: for the treatment of unstable angina hirudin reduces the risk of death and myocardial infarction by 17 %, but at the cost of an increased rate of bleeding. Bivalirudin was tested both as adjuvant therapy in the context of thrombolysis and in the context of periinterventional anticoagulation for interventional therapy in acute coronary syndrome. Compared with heparin plus a GP IIb/IIIa inhibitor, bivalirudin was associated with similar efficacy but a reduced bleeding rate in patients with non–ST-elevation infarction.
Studies with the thrombin inhibitors desirudin, lepirudin, and argatroban are ongoing. Direct thrombin inhibitors are currently recommended primarily as an alternative for patients with known heparin-induced thrombocytopenia.
Pentasaccharides
Pentasaccharides have a chemical structure similar to the AT-III binding site of heparin. Pentasaccharides inhibit factor Xa and thus reduce thrombin formation; they do not directly inhibit thrombin. The major representative of this class is fondaparinux. It is characterized by good bioavailability after subcutaneous administration, does not require monitoring, has a relatively long half-life (15–18 hours), and has no cross-reactivity with HIT-associated antibodies. The compound appeared to be efficacious and safe in numerous studies of acute coronary syndrome. However, in the largest trial, OASIS-6, an increased risk for thrombus formation in and at the catheter system was observed. Therefore, when using fondaparinux during catheter interventions the concomitant use of unfractionated heparin is recommended.
Aspirin
Aspirin (acetylsalicylic acid, ASA) inhibits the biosynthesis of thromboxane (TXA2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


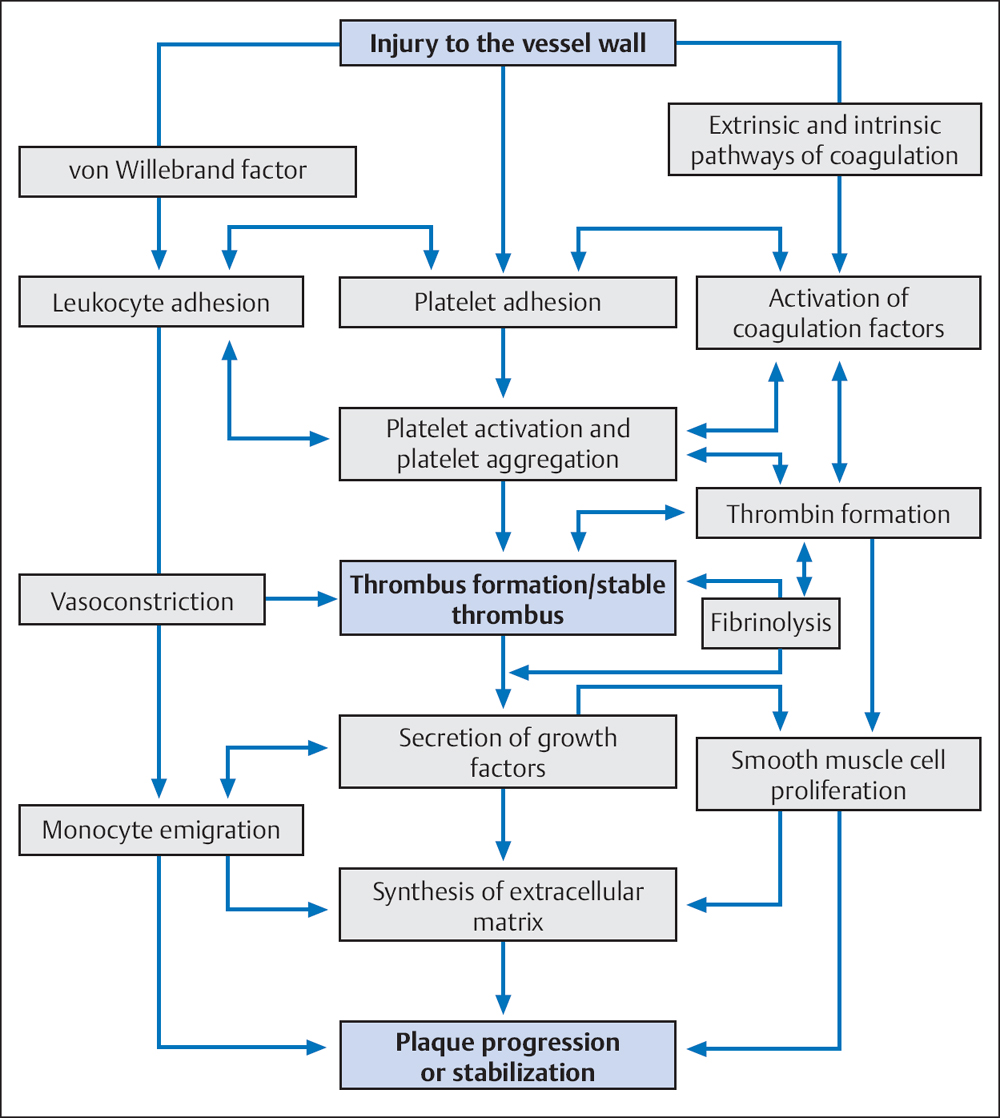
 The second mechanism of platelet activation starts with the release of mediators from the injured vessel wall (von Willebrand factor [vWF], collagen), which lead to platelet adhesion to the endothelial lesion. Platelet adhesion in turn activates further platelets by various mechanisms. Activated platelets mediate numerous processes, for example, vasoconstriction, activation of other platelets, platelet aggregation by fibrinogen bridges via glycoprotein (GP) IIb/IIIa receptors, and the activation of coagulation factors. These processes promote primary hemostasis on the one hand and coagulation on the other.
The second mechanism of platelet activation starts with the release of mediators from the injured vessel wall (von Willebrand factor [vWF], collagen), which lead to platelet adhesion to the endothelial lesion. Platelet adhesion in turn activates further platelets by various mechanisms. Activated platelets mediate numerous processes, for example, vasoconstriction, activation of other platelets, platelet aggregation by fibrinogen bridges via glycoprotein (GP) IIb/IIIa receptors, and the activation of coagulation factors. These processes promote primary hemostasis on the one hand and coagulation on the other.