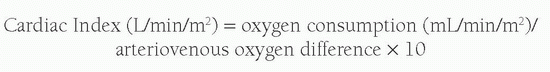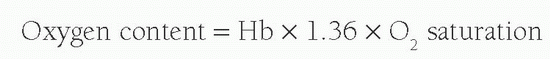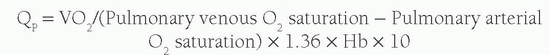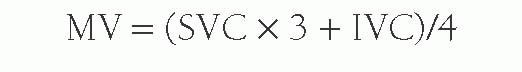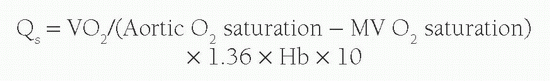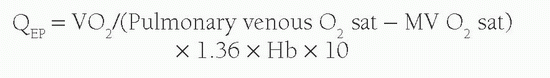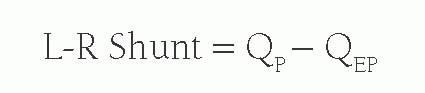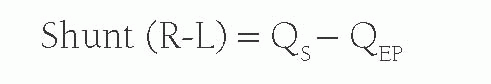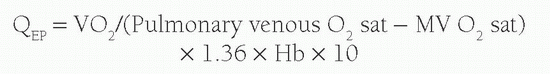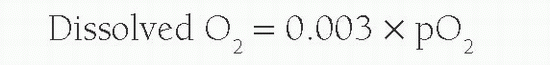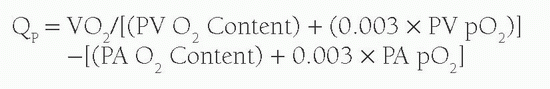Flow and Shunt Calculations
A variety of techniques have been employed to calculate relative right and left heart cardiac outputs and intracardiac shunts; however, most laboratories now use simple oximetric methods. The foundation of these calculations is based upon Fick’s principle, which suggests that the total uptake of a substance by an organ is equal to the product of the blood flow to that organ and the arterial-venous (A-V) concentration difference (gradient) of the substance. When calculating cardiac output, the substance measured is oxygen. In estimating Qp or pulmonary blood flow, the A-V difference used is the difference between the oxygen content of the pulmonary artery and that of the pulmonary veins. In calculating Qs or systemic blood flow, the A-V difference is the difference between the oxygen content of the aorta and that of the systemic veins. Simply put, the equation reads:
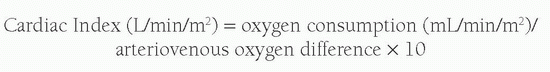
As almost all oxygen in the blood is bound to the hemoglobin and each gram of hemoglobin is capable of carrying 1.36 mL of O2, the oxygen content of blood is calculated by:
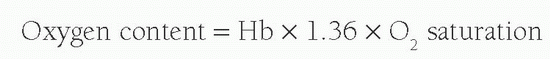
Therefore, the arteriovenous oxygen content difference can be calculated by measuring the oxygen saturations in both the pulmonary arteries and the pulmonary veins. In order to calculate the cardiac output, oxygen consumption is required. This is influenced by a number of variables, including age, sex, height, and heart rate. Accurate measurement during cardiac catheterization is achievable using indirect calorimetry or mass spectrometry; however, it is commonplace to estimate VO
2 values from published predictive equations or tables. It is noteworthy that studies have been published indicating poor levels of agreement between measured and estimated VO
2 (from regression equations published separately by Lafarge and Miettinen, Lundell, Wessel, and Lindahl) in both sedated and mechanically ventilated patients with CHD during cardiac catheterization (
4). Thus Q
p can be calculated from the equation:
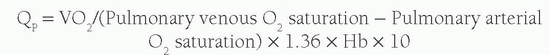
In the absence of any shunt, the pulmonary blood flow and the systemic blood flow should be the same. The systemic blood flow represents the flow from the aorta through the body to the right atrium (RA). Unfortunately, there is a normal variation in the saturation of blood arriving to the RA; the inferior vena caval (IVC) blood has higher oxygen content than the superior vena caval (SVC) blood because the kidneys remove far less oxygen relative to their degree of perfusion. The coronary sinus has very desaturated blood owing to the high oxygen extraction rate of the heart, but the amount of coronary sinus blood is minor, and its contribution is ignored in the equations. The IVC saturation is high enough normally that one needs at least a 11% step-up in the saturation from the SVC to the RA to be sure there is an atrial level shunt. As the blood mixes further downstream in the right ventricle (RV), a 7% step-up in the RV versus the SVC should be used to confirm a ventricular level shunt, and a 5% step-up is recommended in the PA to be confident of a pulmonary arterial left-to-right shunt. To normalize for the higher IVC oxygen content, a mixed venous (MV) saturation is derived from the formula:
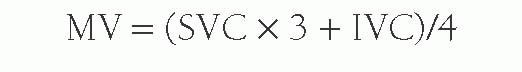
Under normal circumstances with no intracardiac shunting, the aortic oxygen content should be the same as the PV oxygen content, and the MV oxygen content should be the same as the PA oxygen content. Therefore Qs is derived from:
Another concept used with calculation of shunt volume is that of effective blood flow (QEP). The QEP is the volume of desaturated systemic venous blood flowing through the lungs that is oxygenated in the lungs. In the absence of intracardiac shunting, the effective pulmonary blood flow (QEP) should be the same as the QP, which should be equal to Qs:
However, when there is a left-to-right shunt, the shunted blood has already passed through the lungs, and is therefore not part of QEP. Therefore, the volume of shunted blood is calculated by:
When there is a right-to-left shunt, QS will be formed by both the QEP and the blood that has shunted past the lungs. Therefore, the right-to-left shunt volume is calculated by:
A simpler method for assessing the systemic to pulmonary flow ratios can be calculated with the O2 saturations from the four main measurement areas used in the above calculations, namely the MV saturation, the pulmonary artery saturation, the pulmonary vein saturation, and the aortic saturation as in calculating relative QP:QS as all of the other variables will cancel each other out. Therefore, this can be calculated roughly by:
The above equation will work in providing an estimate of leftto-right shunt in most cases. However, if oxygen concentrations higher than room air are used, the dissolved oxygen content per milliliter of blood is required for accurate calculation flow. This is calculated from:
This is then incorporated into the calculations:
Calculation of Pulmonary Vascular Resistance
With invasive measurements, exact pressure recordings from the heart, pulmonary arteries, and the aorta can be obtained. It is also possible as outlined above to calculate cardiac output. Therefore, by applying Ohm’s law (pressure = flow × resistance), one can calculate accurate resistance measurements, particularly within the pulmonary arteries. This is extremely important as pressure may be elevated secondary to increased flow or increased resistance.
If flow is the predominant factor, such as in the case of a large ventricular septal defect (VSD) in a young infant, then surgical management is indicated. However, if distal irreversible pulmonary vessel remodeling occurs (which may be seen with chronic excessive pulmonary flow), then high resistance may preclude surgical intervention. This provides the cornerstone of diagnostic catheter assessment in CHD patients, where there are concerns regarding the contributing factors to increased pulmonary artery pressures. Pulmonary vascular resistance (PVR) is calculated by modifying Ohm’s law:

Similarly, the systemic vascular resistance (SVR) is derived from the mean aortic pressure, the mean RA pressure, and the systemic blood flow.
When the flow is expressed in terms of liters per minute, the units derived are referred to as Hybrid or Wood Units and measured in mm Hg/L/min, or simply referred to as “u”. It is important to note that these values when indexed to body surface area are expressed as u·m
2. Smaller patients appear to have significantly greater resistance values when using nonindexed cardiac output values (because of their smaller body surface areas [BSA]), and therefore it is important to multiply, rather than divide, by the BSA to calculate the indexed resistance value. The Woods Unit number can be multiplied by 8 to convert to Pascal seconds per cubic meter (MPa·s/m
3) and by 80 to derive dynes cm/s
5. These values are outlined in
Table 44-2.
Although initial assessment of vascular resistance may suggest significantly raised PVR, it is possible that this increased resistance may be reversible and therefore react favorably to certain pulmonary vasodilators. Such agents include oxygen and pulmonary vasoactive drugs such as adenosine, calcium-channel blockers, prostacyclin, and inhaled nitric oxide. A “positive” response is generally one in which there has been both a reduction in mean PA pressure of at least 10 mm Hg to an absolute mean PA pressure of less than 40 mm Hg without a decrease in cardiac output (
5). A positive response has been associated with an improved longterm outcome, although the response may not predict clinical improvement with the various pulmonary hypertensive agents now available.
Eisenmenger’s physiology is defined by the presence of pulmonary hypertension and evidence for shunt reversal (cyanosis), which occurs because of increased distal PVR secondary to chronic excessive blood flow. Initial assessment and management protocols for these patients have been defined (
2). Significant symptom improvement has been shown following treatment with newer oral pulmonary hypertensive agents (
6).