22 Percutaneous Coronary Intervention and Coronary Stent Implantation A milestone in modern medicine was the introduction in 1977 by Andreas Grüntzig of percutaneous coronary intervention (PCI; this term encompasses balloon angioplasty with and without stent implantation as well as other interventions such as atherectomy; the term “percutaneous transluminal coronary angioplasty” (PTCA) is now also referred to as “plain old balloon angioplasty” (POBA)). The first nonsteerable balloon catheters were stiff and had thick walls, so that initially only proximal stenoses could be reached. The balloons, which were made from noncompliant material, would burst at an inflation pressure of only 6 atm (600 kPa). Significant problems of sole PCI balloon angioplasty were the high rates of peri-interventional acute vessel occlusions, usually due to dissection, and restenosis. Another milestone in the further development of PCI was therefore the introduction of coronary stents in the mid-1980s. However, further improvements were required for the breakthrough of the methodology: intensified pharmacological platelet inhibition, high-pressure implantation, and, to reduce the rate of restenosis, the development of drug-eluting stents (DES). With the steady improvement in catheter and stent materials, the optimization of steerable coronary guidewires, the increasing experience of the operators, and the development of evidence-based treatment strategies, PCI has developed into the primary therapy for coronary revascularization. Even coronary lesions that are complex or difficult to access such as bifurcations and chronic total occlusions can be treated relatively safely and with a high success rate by PCI. The use of PCI has substantially broadened over time. The current methodologies in the interventional treatment of stable coronary artery disease are presented below. The extended technical possibilities, the clinical results, and the safety of coronary angioplasty continuously change the indications for PCI versus coronary artery bypass graft (CABG) surgery. The basis for both indications is overt or inducible ischemia in the area supplied by one or more coronary arteries that can be revascularized and preserved myocardial viability in that area. Prognostic and symptomatic indications are differentiated on this basis. The following criteria are important when considering PCI: – EuroScore II (http://euroscore.org) and/or – STS Score (http://riskcalc.sts.org) Classic indications for surgical myocardial revascularization continue to be coronary three-vessel disease as well as left coronary main stem stenosis, and for PCI coronary one- or two-vessel disease. However, due to advances in interventional cardiology, today left main stem stenosis and coronary three-vessel disease also belong to the standard repertoire of an experienced interventional center, especially in patients with high perioperative risk. Table 22.1 Absolute and relative contraindications for PCI
Percutaneous Coronary Intervention (PCI)
 Introduction
Introduction
 Indications/Contraindications
Indications/Contraindications
 Absolute and relative contraindications (Table. 22.1)
Absolute and relative contraindications (Table. 22.1)
 The individual risk of PCI as compared with the risk of surgical revascularization (Tables 22.2 and 22.3)
The individual risk of PCI as compared with the risk of surgical revascularization (Tables 22.2 and 22.3)
 Completeness of revascularization with multivessel disease (PCI versus CABG)
Completeness of revascularization with multivessel disease (PCI versus CABG)
 Restenosis rate after PCI (clinical and angiographic factors, Table 22.4)
Restenosis rate after PCI (clinical and angiographic factors, Table 22.4)
 Likelihood of one or more reoperations or reinterventions, especially in younger patients
Likelihood of one or more reoperations or reinterventions, especially in younger patients
 In older patients (> 75 years) the markedly increased surgical risk
In older patients (> 75 years) the markedly increased surgical risk
 Comorbidities, especially chronic kidney disease, diabetes, stroke, immobility, and frailty The risk can be assessed with
Comorbidities, especially chronic kidney disease, diabetes, stroke, immobility, and frailty The risk can be assessed with
– High interventional risk due to anatomy (example: distal stenosis of the left coronary main stem or a main stem equivalent with involvement of the ostia of the LAD and the LCX) – Type C stenoses with expected success rate < 60 % – Hemodynamically insignificant coronary stenosis < 50 % without inducible ischemia – No clinical signs of ischemia and functional tests negative for ischemia or invasive fractional flow reserve (FFR) > 0.75 – Lack of bail-out options, lack of surgical standby – High morbidity relative to the expected clinical benefit – Inadequate radiography equipment – Inadequate experience in complex interventions and their postinterventional care |
Table 22.2 Risk factors for complications associated with coronary intervention
Clinical factors | – Female sex – Unstable angina – Inadequate inhibition of platelet aggregation or contraindication for adequate dual platelet inhibition – Diabetes mellitus – Chronic kidney disease – Peripheral vascular anatomy with risk for postinterventional bleeding complications |
Angiographic factors | – Type B2 and type C lesions (see Table 16.1), especially – Angulations ≥ 45 – Bifurcation stenosis – Severe calcifications – Long lesions – Intracoronary thrombus – Soft atheroma core – Stenoses of the right coronary artery – Degenerated vein graft |
Procedural factors | – Residual stenosis > 50 % – Residual dissection > type B and > 10 mm – Oversized balloon catheter – Insufficient stent expansion – Residual transstenotic pressure gradient > 20 mm Hg, FFR < 0.9 |
Table 22.3 Risk factors for increased morbidity and mortality associated with coronary intervention
Clinical factors | – Female sex – Unstable angina pectoris – Advanced age – Heart failure – Chronic kidney disease – Peripheral arterial disease – Bleeding risks |
Angiographic factors | – Atherosclerosis of the left coronary main stem – 3-vessel disease – Ejection fraction < 0.30 – Contralateral akinesia – Ostial stenosis of the right coronary artery – Stenosis in a vessel that feeds collaterals – Last open coronary artery – Epicardial collaterals for retrograde access during CTO interventions |
Due to the various manifestations of CAD, the diverse presentations of individual patients, and the ongoing evaluation of different approaches, a specific outline of the use of the therapeutic options currently considered appropriate is beyond the scope of this book. The reader is referred to current guidelines and appropriate use criteria provided by appropriate professional bodies. Nevertheless, decisions have to be made on a case-by-case basis. The immediate technical feasibility is not the crucial factor, but rather the acute and long-term prospects of success of the revascularization method to maximize patient benefit.
Table 22.4 Risk factors for restenosis after PCI
Clinical factors | – Acute coronary syndrome – Angina pectoris of short duration (> 2 – 6 months) – Diabetes mellitus – End-stage renal failure requiring dialysis, especially with hyperparathyroidism |
Angiographic factors | – Long lesions (> 15 mm) – Small diameter of the stenotic coronary artery (≤ 2.7 mm) – Vein grafts – Recanalized chronic total occlusion – Ostial stenosis – Bifurcation stenosis – Stenosis in bend (> 45°) – Severe stenosis before PCI |
Procedural factors | – Residual stenosis > 30 % – Pressure gradient after PCI > 20 mm Hg or FFR < 0.9 |
The SYNTAX score provides guidance for the treatment of coronary lesions based on their characteristics (http://syntaxscore.com.)
 Sole Balloon Angioplasty (“Plain Old Balloon Angioplasty”)
Sole Balloon Angioplasty (“Plain Old Balloon Angioplasty”)
PCI is usually completed with stent implantation. POBA will be described first as some principles of PCI can be illustrated here.
On the basis of ultrasound studies and pathological findings, four mechanisms appear to be mainly responsible for the dilatation of the lumen after balloon angioplasty:
1. Overdilatation of the vessel wall with increase in the outer vessel diameter
2. Plaque compression
3. Plaque rupture with tearing of the intima and/or segments of the media (dissection)
4. Remodeling of the vessel wall
Depending on the composition (soft, lipid-rich versus calcified, fibrotic plaque) and localization of the plaque in the vessel (concentric versus eccentric), the lumen gain is achieved by different mechanisms. For example, concentric, fibrotic or calcified atheromas tend to dissect, whereas the lumen gain in eccentric, lipid-rich plaques is predominantly due to plaque compression and overdilatation of the contralateral vessel wall.
 Equipment
Equipment
The equipment for performing POBA is shown in Fig. 22.1.
Guiding Catheter
Selection of the correct guiding catheter is an essential factor in successful coronary intervention. It should fulfill the following requirements.
 It should be as atraumatic as possible.
It should be as atraumatic as possible.
 It should maintain stability even during interventions of longer duration.
It should maintain stability even during interventions of longer duration.
 It should have a large lumen to also allow double-balloon maneuvers and so on.
It should have a large lumen to also allow double-balloon maneuvers and so on.
Even though individual, specific catheter configurations have been developed for interventional catheterization, most guiding catheters have the same shape as catheters for diagnostic coronary angiography. The catheters usually have an internal, and at times also external hydrophilic coating. A middle layer of braided Kevlar fibers provides the necessary stiffness and torque response of the guiding catheter, maintains the shape of the catheter during longer-lasting interventions, and increases the backup when advancing the balloon catheter. To avoid injury to the ostium, guiding catheters have a soft tip, which at times is specifically radiopaquely marked.
Compared with diagnostic catheters, guiding catheters have a different structure to guarantee sufficient stability with providing the largest lumen possible and a thin wall (Fig. 22.2a). The wall is usually supported by a wire mesh. The design of the distal curve varies to fulfill the requirements for both sufficient flexibility and adequate stability (Fig. 22.2b).
Guiding catheters with side holes can be used to maintain a residual perfusion in vessels with small calibers and ostial stenoses, which can be occluded by the catheter. However, when using such catheters it should be kept in mind that, despite an undamped pressure wave, there is only limited antegrade perfusion. Furthermore, coronary opacification is somewhat decreased due to the contrast flow into the aorta via the side holes and therefore the amount of contrast medium required is increased.
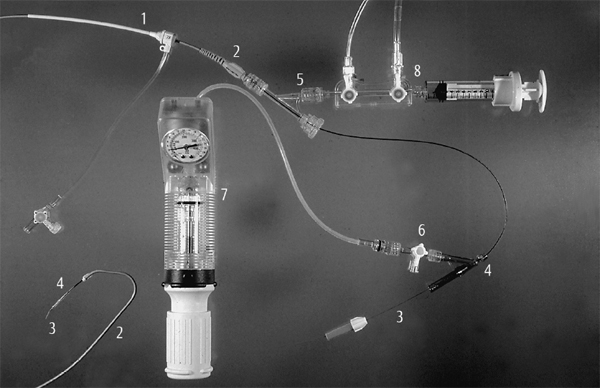
Fig. 22.1 Equipment to perform a PCI.
1 Sheath
2 Guiding catheter
3 Guidewire
4 Balloon
5 Y-connector
6 High-pressure stopcock
7 Inflation syringe
8 Manifold
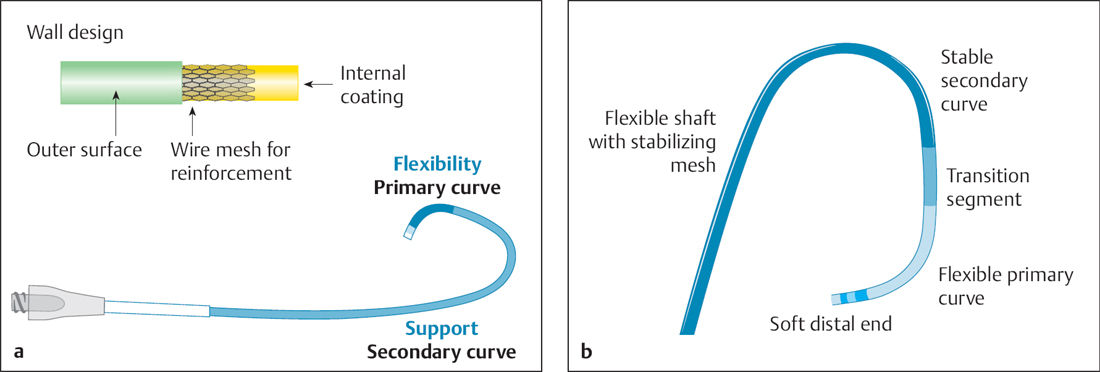
Fig. 22.2 a, b Structure of a guiding catheter.
a The wall is stabilized by an incorporated wire mesh, so that the lumen gain is primarily due to a reduced wall thickness.
b Illustration of the different segments of the distal end of a guiding catheter.
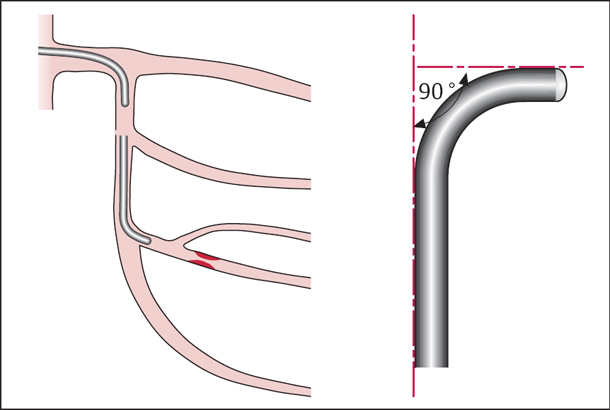
A catheter shape should be selected that will achieve an ideally axial orientation between guiding catheter and coronary ostium and provide a good backup against the contralateral aortic wall.
Left coronary artery. For the left coronary artery a special extra support curve (EBU, etc.) is most often used. The classic Judkins shape can also be considered, especially for LAD interventions. A good projection for coronary interventions to evaluate the origins of the LAD and LCX from the left main stem is the 40 to 50° LAO projection with caudal angulation (so-called “spider view”).
 If the main stem is short and a PCI of an LAD stenosis is planned, both engagement with the guidewire and subsequent advancement of the balloon are easier when a smaller Judkins curve (JL 3.5) is selected. The same is true if the LAD takes off from the stem at a steep angle.
If the main stem is short and a PCI of an LAD stenosis is planned, both engagement with the guidewire and subsequent advancement of the balloon are easier when a smaller Judkins curve (JL 3.5) is selected. The same is true if the LAD takes off from the stem at a steep angle.
 For the LCX a Judkins 4 curve is usually suitable, which can be directed to the LCX origin by clockwise rotation.
For the LCX a Judkins 4 curve is usually suitable, which can be directed to the LCX origin by clockwise rotation.
 Improved back-up for PCI of the LCX (distal stenosis, tortuous vessel) can be obtained in particular with the left Amplatz guiding catheter, which most often selectively engages the LCX ostium.
Improved back-up for PCI of the LCX (distal stenosis, tortuous vessel) can be obtained in particular with the left Amplatz guiding catheter, which most often selectively engages the LCX ostium.
For main stems with small diameters that are partially or completely occluded by the guiding catheter (as indicated by ventricularization of the pressure curve after seating), all guiding catheters are also available with side holes.
Right coronary artery. For the RCA the Judkins right curve is most often selected. Back-up can be increased by using the right or left Amplatz catheter. For a “shepherd’s crook” configuration of the RCA the left Amplatz catheter or an internal mammary guiding catheter are suitable. Many operators always use guiding catheters with side holes for the RCA (occlusion of the ostium by the guiding catheter is frequently more pronounced than with the LCA, longer dwelling time in the vessel compared with coronary angiography, repeated contrast media injection, which can trigger ventricular fibrillation with forceful injection).
 Guiding catheters with side holes require more contrast media—this is of significance especially for patients with chronic kidney disease.
Guiding catheters with side holes require more contrast media—this is of significance especially for patients with chronic kidney disease.
Balloon Catheter
A variety of catheter types for coronary angioplasty are illustrated in Fig. 22.3.
Over-the-wire (OTW) balloon catheter. The double-lumen catheter is moved in its entire length (155 cm) over a coronary guidewire and is therefore characterized by good pushability. However, the system requires the use of long coronary guidewires (300 cm), the (cumbersome) extension of shorter guidewires, or trapping methods for the short guidewires in case a balloon exchange is required with the guidewire in place. In addition, the catheter exchange has to be done under fluoroscopy. These catheters are sometimes used for the recanalization of chronic occlusions but also, for example, for transcoronary ablation of septal hypertrophy (TASH)/percutaneous transluminal septal myocardial ablation (PTSMA). Via the wire lumen of an OTW balloon, distal segments of a vessel can be visualized or reached with an injectate.
Rapid-exchange system (monorail system). These systems are currently standard for conventional PTCA and for premounted stents. The wire lumen in this system does not extend through the entire length of the catheter, but enters the catheter ~17 to 40 cm from the distal end. The remainder of the wire is next to the catheter shaft. Thus, the balloon catheter is advanced and retracted as if on a monorail. The system has the advantage of short guidewires with the capability of rapid catheter exchange without fluoroscopy. When advancing the system in the guiding catheter, the operator has to take care that the introduced guidewire and the balloon catheter are advanced simultaneously. Otherwise, the coronary guidewire could slip out of the short wire lumen, and thus the balloon would lose its wire guidance. It is worth noting that the steerability of the guidewire is somewhat reduced compared with the over-the-wire system, which can be a disadvantage especially when trying to recanalize a chronic occlusion.
Cutting balloon and scoring balloon. A cutting balloon consists of a noncompliant monorail balloon that is coated with two or three longitudinal knives on the balloon material. A scoring balloon is coated with longitudinal or helical wires. The cutting balloon is discussed separately in Chapter 24. These systems generally have the advantage that they do not slip out of position (particularly important for PCI of in-stent stenoses) and do not cause uncontrolled dissections.
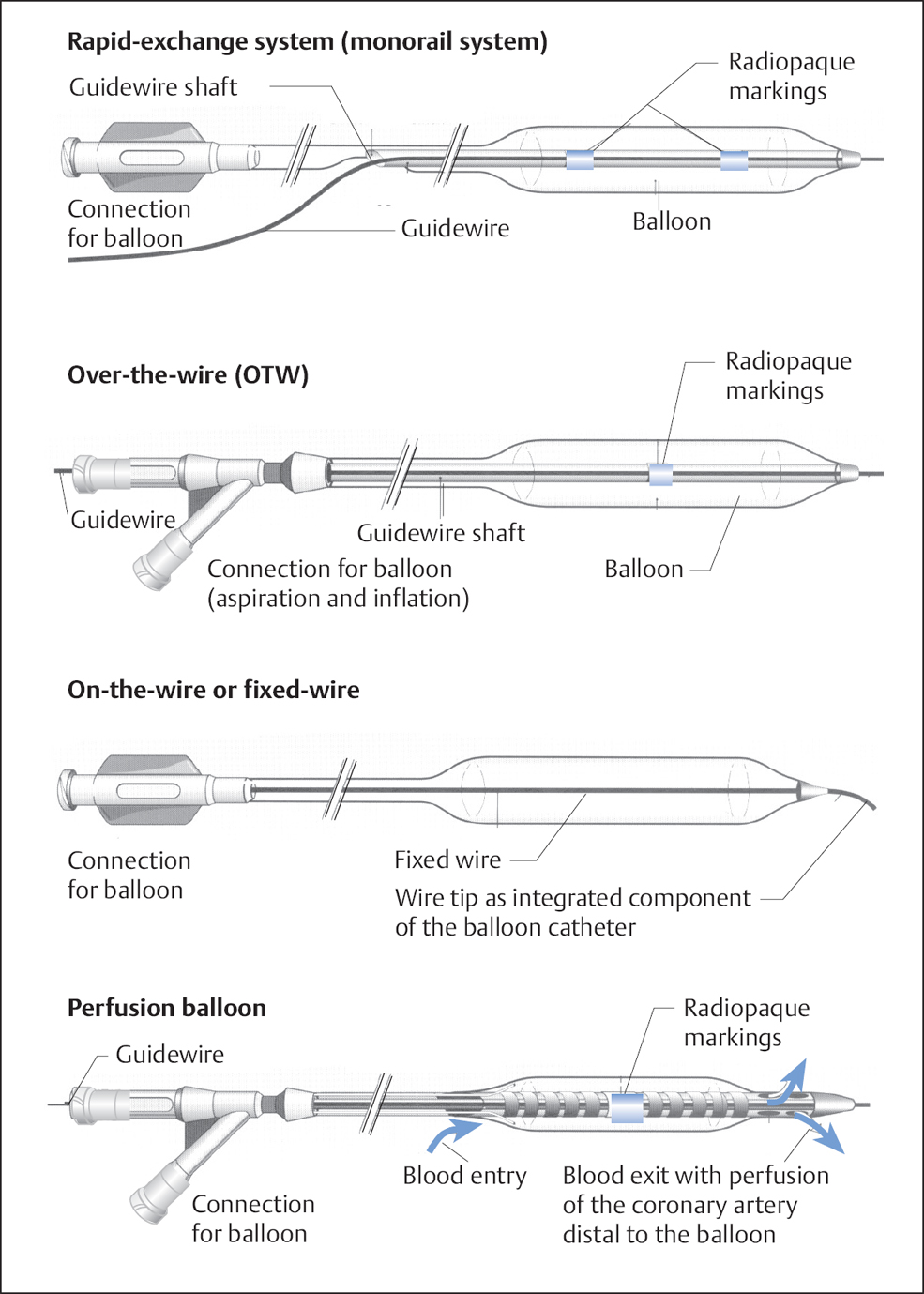
Fig. 22.3 A variety of balloon catheters for coronary angioplasty. On-the-wire balloon catheters and perfusion catheters are no longer of clinical relevance.
Drug-eluting balloons (DEBs). Balloons can also be used to locally deliver antiproliferative drugs at the time of PCI. Paclitaxel-eluting balloons are a mainstay in the interventional treatment of in-stent restenoses or POBA of small vessels that do not appear suitable for stent implantation. Different products use different carrier substances to fix paclitaxel on the balloon then deliver it to the vessel wall during longer balloon inflations of > 60 seconds.
Hydrophilic iopromide and also urea and crystalline structures such as shellac have been used as a carrier for paclitaxel. The greatest experience is available for DEBs that use hydrophilic iopromide as carrier.
On-the-wire balloon catheters and perfusion catheters. On-the-wire balloon catheters and perfusion catheters are no longer of clinical relevance.
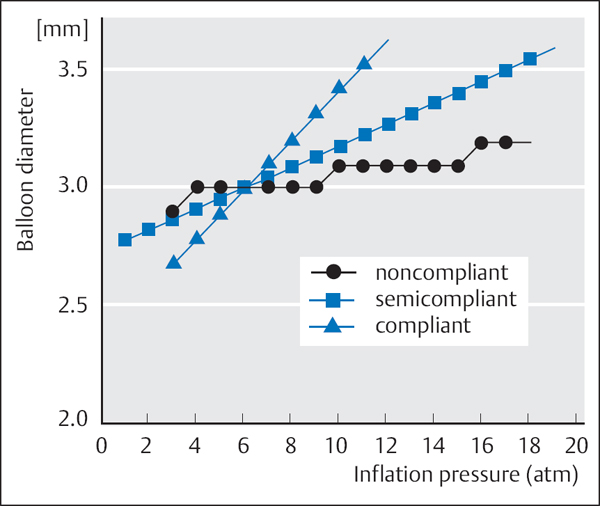
Balloon material. Balloon catheters continue to be improved and are quite different from the early noncompliant PVC balloons, which were stiff and had a large profile. Catheters are offered with different balloon diameters (1.0 – 5.0 mm), different balloon lengths (8 – 40 mm), and, depending on the balloon material, different compliances (Fig. 22.4).
The following are differentiated depending on the material:
 Noncompliant (or low-compliance) (material: plastomer PM 300)
Noncompliant (or low-compliance) (material: plastomer PM 300)
 Semicompliant (material: polethylene PE 600, polytetrafluorethylene, or polyurethane)
Semicompliant (material: polethylene PE 600, polytetrafluorethylene, or polyurethane)
 Compliant (material: polyolefin copolymer POC)
Compliant (material: polyolefin copolymer POC)
A noncompliant balloon changes its diameter only slightly with increasing dilatation pressure, and thus has the advantage that the vessel is not overdilated even when high pressure is applied. Depending on the pressure, differences in diameter of almost 1 mm can occur with compliant balloons; thus, high inflation pressures can result in overdilatation of the coronary artery. However, these balloons have the advantage that they can adjust to variable sizes during the dilatation. Compliant balloons are no longer in clinical use. In general, semicompliant balloons are employed, which represent a compromise between the two strategies.
Criteria for the selection of the balloon material are shown in Table 22.5.
Balloon preparation
All air has to be removed from the system. The balloon is prepared in a similar way for all catheters: The lumen is flushed with heparinized normal saline: from the proximal end for OTW catheters, and from the distal end for monorail systems. Using a 10- to 20-mL syringe with an attached three-way high-pressure stopcock (filled with ~3–4 mL of a 1:1 mixture of contrast medium and normal saline), the catheter is repeatedly flushed until all air has been removed. After the last aspiration, the balloon is filled with a small amount of contrast by releasing the pull on the plunger of the syringe, and the balloon connection is subsequently occluded with the three-way stopcock. The coronary guidewire is introduced from the proximal end for OTW catheters and retrogradely from the tip for monorail catheters.
Table 22.5 Criteria for selecting the balloon material
Semicompliant balloon | Noncompliant balloon |
Uncertain vessel diameter Multiple stenoses Stenoses in bends | Hard or calcified stenoses Elective stent implantation Repeat dilatation of a not fully expanded stent Rupture of a semicompliant balloon |
During stent implantation we do these maneuvers only after the stent has already been advanced to the coronary artery to prevent a possible loosening of the stent from the balloon.
Coronary Guidewires
The coronary stenosis is crossed with a steerable guidewire. The flexible, shapeable tip consists of a thin beveled guidewire end, which is covered with a fine spring coil of radiopaque material. Three different wire tips are distinguished:
1. Floppy: a soft, atraumatic tip
2. Intermediate: compared with floppy it has a somewhat firmer tip and is thus more steerable
3. Standard: a firm and thus more traumatic tip, but with good steerability
Guidewires have a length of 190 cm or 300 cm and are available in three sizes (0.010 in., 0.014 in., 0.018 in.). For the 190 cm-long wire, a 122 cm-long extension wire is available. Usually a 0.014-in. wire is used for routine PCI. Guidewires with hydrophilic or hydrophobic coating as well as polymer-coated tips are available for special indications.
Special wires are used to recanalize chronically occluded arteries; their characteristics will be discussed separately.
Usually, a wire has a core of stainless steel or nitinol, which provides stability. The tip is covered with wire or polymer so that it can be shaped (Fig. 22.5).
For indications that require stronger support, intermediate or floppy tipped wires are available that have increased support in the proximal segments of the wire (“extra support”). For the recanalization of chronic occlusions, there are also specific wires that differ in their degree of stiffness and tip design. Sometimes the wires are marked at defined distances, which allows for the measurement or estimation of the length of a coronary lesion. The wires are radiopaque either throughout the length of the wire or only at the tip.
The most important characteristics of a coronary guidewire are summarized in Table 22.6.
Coronary guidewires consist of alloy steel, nitinol, or a combination of both materials. Table 22.7 summarizes how steel and nitinol affect the characteristics of the wire.
Personal view
To optimize interventional success, we believe it is more important for an operator to be familiar with the characteristics of a few selected wires for specific indications (routine, chronic occlusions, “extra support”), rather than to stock the entire spectrum of commercially available wires in the catheterization laboratory.
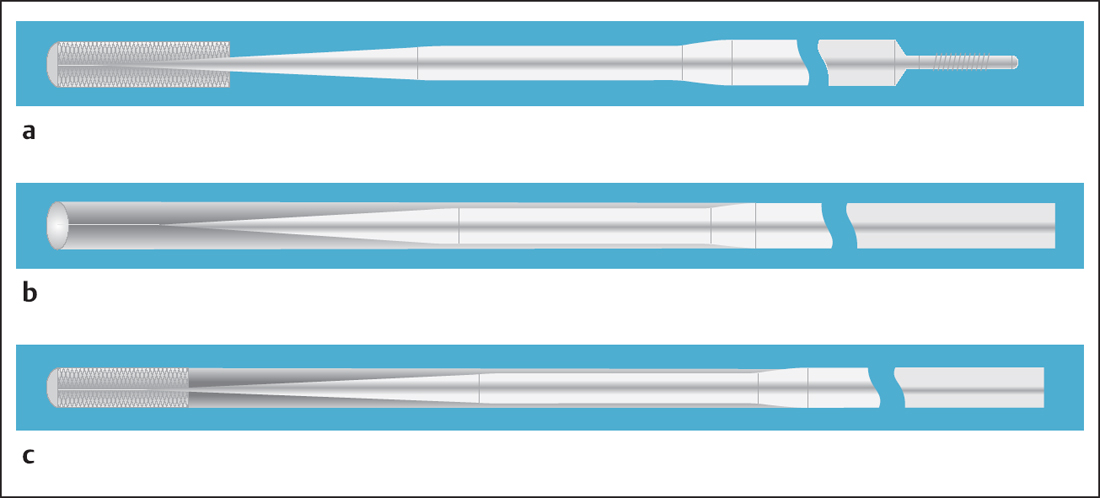
Fig. 22.5 a–c Design of coronary guidewires.
a Tip with coated core and changing thickness results in variable flexibility of the tip.
b Tip and coating made of polymer facilitate the crossing of calcified lesions.
c Spiral-coil covered tip and polymer coating result in high “glide-ability” with an atraumatic tip.
Table 22.6 Characteristics of a coronary guidewire
Wire functions | Wire characteristics |
– Access to the target vessel – Crossing of the target lesion – Support for balloon, stent, etc. | – Torque, i. e., 1:1 transmission of wire shaft rotation to the wire tip – Control of the tip – Shapeability of the tip – Trackability – Steerabilty – Stiffness (resistance to kinking) – Visibility (of the tip) – Tactile feedback – Adjustment to the vessel course or straightening of the vessel – Flexibility – “Glide-ability” |
Table 22.7 Comparison of the characteristics of steel and nitinol
Steel | Nitinol |
– Hardness – Flexibility – Stability of the tip configuration – Better torque – Better tactile feedback – But: – More susceptible to kinking | – Elasticity – Greater flexibility – Memory of the previous shape also after kinking – Less kinking – Less torque – But: – Less tactile feedback |
Prior to the intervention, the wire tip is preshaped corresponding to the coronary anatomy and the stenosis morphology. This is done either alone with the fingers or by careful bending of the tip over a cannula. Usually, the end of the wire is curved so that the wire tip is perpendicular to the wire shaft (Fig. 22.6). The radius of the curve depends on the vessel diameter and the angulation of the coronary vessel.
 General Requirements Prior to Coronary Interventions
General Requirements Prior to Coronary Interventions
Surgical Standby
With the modern procedures of interventional cardiology the immediate standby of a cardiac surgeon during PCI is no longer required. Due to improved technique and the introduction of stents, the frequency of emergent CABG has decreased substantially (0.4 – 1 %). Coronary angioplasty without direct standby of a surgical team is generally safe and has become common clinical practice.
However, ultimately even a presumably “simple” coronary angioplasty can take an unexpected course that cannot be managed interventionally. Therefore, coronary angioplasties should in general be done in close cooperation with a cardiosurgical department at a highly experienced interventional center. Ideally, though not absolutely required, cardiac surgery should be in the same location as the catheterization laboratory. If not, then it should be within 30 minutes travel.
High-risk interventions should only be performed by highly experienced operators with all bail-out options available. The risk assessment and therefore also the immediate responsibility are with the operator. Thus, it is required to have in place specific bail-out strategies (see p. 256) that require close cooperation with a cardiac surgery department.
Requirements Regarding Equipment and Personnel
Required equipment. In terms of equipment required in a catheterization laboratory for coronary interventions, it is particularly important to consider the possible need for advanced life support. For example, the laboratory has to be large enough to accommodate a ventilator a cardiac ultrasound platform, and of course the necessary personnel must be available to provide the corresponding intensive care. It would be advantageous if the cardiac catheterization laboratory were in the immediate vicinity of the ICU or CCU of the clinic. In addition, sufficient appropriate equipment has to be in stock to manage all possible complications of the coronary intervention. The special requirements for the radiographic and imaging technology used during coronary interventions have been discussed in Chapter 4 under Radiation Technology and Radiation Safety (p. 3).
Requirements regarding personnel and training. The specific requirements for working in diagnostic or interventional cardiology or to run a cardiac catheterization laboratory differ from country to country. The best training is generally obtained in centers with a large number of interventions and by years of instruction and cooperation in an experienced team. This imparts the relevant knowledge regarding indications, different interventional approaches, and the management of less common complications. To maintain skills already obtained, it is recommended to carry out at least 50 interventions per year. That these should be considered minimal requirements is supported by the observation that both procedural failure and the rate of complications increases with lower rates of interventions.
Monitoring after Intervention
Because of the risk of postinterventional complications after an initially successful PCI, patients should be monitored for ~24 hours. Furthermore, in a patient with an acute coronary occlusion it should be possible to carry out without delay a repeat coronary angiography and intervention (24-hour standby). Whether monitoring is done in the ICU or CCU or on a regular ward depends on the original indication (elective, unstable angina, or acute myocardial infarction) and on the result of the intervention. The operator is responsible for appropriate postprocedural care and, if necessary, for required emergency treatments.
Personal view
We consider transfer immediately after PCI to a hospital without capability for reintervention to be contraindicated.
 Procedure
Procedure
Specific Patient Preparation
Generally, the same rules apply as for diagnostic cardiac catheterization. Informed consent must specifically include the procedure and risks of PCI, usually with stent implantation. This includes the risk of possible extension of the intervention, the risk of dissection, the possibility of emergency surgery, and so on.
Contrast media. The amount of contrast administered during PCI frequently cannot be anticipated, and in many cases exceeds the expected amount of contrast used for diagnostic cardiac catheterization. Therefore, careful preparation and postprocedural care of patients with chronic kidney disease and impaired ventricular function is required.
Premedication. For elective PCI, premedication with antiplatelet agents is required. Patients not already on chronic ASA (aspirin) should start 81 to 325 mg daily immediately after the indication for PCI has been determined. Pretreatment with clopidogrel reduces the periprocedural rate of ischemic complications. The therapy starts on the day of the intervention with a loading dose of 300 or 600 mg clopidogrel (depending on the time window) or > 3 days before with the maintenance dose of 75 mg. For high-risk patients it should be checked on an individual basis whether administration of a GP IIb/IIIa inhibitor or alternative anticoagulants such as bivalirudin are indicated. Patients at risk for reaction to contrast medium receive the usual preparation (Chapter 3).
Procedure: Balloon Angioplasty
 Puncture of the radial or femoral artery.
Puncture of the radial or femoral artery.
 Placement of a 5, 6, or 7F sheath.
Placement of a 5, 6, or 7F sheath.
 If kinking of the iliac artery is present, long sheaths (up to 22.5 cm) will improve the steerability of the guiding catheter.
If kinking of the iliac artery is present, long sheaths (up to 22.5 cm) will improve the steerability of the guiding catheter.
 Placement of the guiding catheter.
Placement of the guiding catheter.
 Anticoagulation:
Anticoagulation:
– Weight-adjusted administration of a total of 5,000 to 10,000 IU (70–100 IU/kg body weight) unfractionated heparin IV or IC (target ACT: 250 – 350 seconds; less when using a GP IIb/IIIa inhibitor) or
– Enoxaparin IV 0.5 to 1 mg/kg body weight or 0.5 to 0.75 mg/kg body weight with simultaneous administration of a GP IIb/IIIa inhibitor
 ASA 81 to 325 mg (325 mg nonenteric ASA for patients not on ASA therapy); alternatively 0.5 g ASA IV if available.
ASA 81 to 325 mg (325 mg nonenteric ASA for patients not on ASA therapy); alternatively 0.5 g ASA IV if available.
 Nitroglycerin 0.5 to 1.0 mg IC.
Nitroglycerin 0.5 to 1.0 mg IC.
 Imaging of the target lesion in the best projection (no overlap, no foreshortening) in both the RAO and the LAO projections (Table. 22.8). Store still frames on the monitor for reference.
Imaging of the target lesion in the best projection (no overlap, no foreshortening) in both the RAO and the LAO projections (Table. 22.8). Store still frames on the monitor for reference.
Table 22.8 Projections frequently used for PCI according to stenosis location
Right coronary artery (RCA) | Left circumflex (LCX) | Left anterior descending (LAD) |
Proximal | Proximal | Proximal |
LAO 40–60°; caudal 10–20° | LAO 40–50°; caudal 20° | LAO 40–50°; caudal 20° |
RAO 30° | RAO 30° | LAO 90° |
Mid-RCA | LCX/marginal branches | Mid-LAD/diagonal |
LAO 40–60° | LAO 50°; caudal 20° | LAO 50°; cranial 20° |
RAO 30° | RAO 30°; caudal 15–20° | LAO 90° |
Distal RCA | Distal LCX | Distal LAD |
LAO 40–60°; cranial 20° | LAO 40–60°; cranial 20° | LAO 50°; cranial 20° |
| RAO 30°; caudal 20° | RAO 30°; caudal 20° |
 Preparation of the dilatation syringe: about half of the syringe is filled with a 1: 1 mixture of contrast medium and normal saline; the syringe should be purged of air.
Preparation of the dilatation syringe: about half of the syringe is filled with a 1: 1 mixture of contrast medium and normal saline; the syringe should be purged of air.
 Balloon/stent selection with size determination: the balloon/stent diameter should be matched as closely as possible to the diameter of an undiseased portion in the segment of the vessel to be dilated. Either this is done using quantitative coronary angiography (QCA), or the vessel diameter is estimated from a comparison with the size of the guiding catheter placed:
Balloon/stent selection with size determination: the balloon/stent diameter should be matched as closely as possible to the diameter of an undiseased portion in the segment of the vessel to be dilated. Either this is done using quantitative coronary angiography (QCA), or the vessel diameter is estimated from a comparison with the size of the guiding catheter placed:
– 6F = 1.98 mm diameter
– 7F = 2.31 mm diameter
– 8F = 2.64 mm diameter
– 9F = 2.97 mm diameter
 Preparation of the balloon catheter: flushing of the catheter lumen with normal saline; removal of all air from the balloon by aspiration with a 20 mL syringe filled with ~3 to 4 mL of the normal saline–contrast medium mixture.
Preparation of the balloon catheter: flushing of the catheter lumen with normal saline; removal of all air from the balloon by aspiration with a 20 mL syringe filled with ~3 to 4 mL of the normal saline–contrast medium mixture.
 Introduction of the coronary guidewire into the balloon catheter. The tip of the guidewire is preshaped to correspond to the vessel course and then pulled back into the tip of the balloon catheter.
Introduction of the coronary guidewire into the balloon catheter. The tip of the guidewire is preshaped to correspond to the vessel course and then pulled back into the tip of the balloon catheter.
 Balloon catheter and guidewire are introduced together via the Y-connector into the guiding catheter and advanced until shortly before the coronary ostium.
Balloon catheter and guidewire are introduced together via the Y-connector into the guiding catheter and advanced until shortly before the coronary ostium.
 With the aid of a torque tool, the guidewire is advanced under fluoroscopy without pressure by rotational movements across the stenosis into the periphery of the stenotic vessel.
With the aid of a torque tool, the guidewire is advanced under fluoroscopy without pressure by rotational movements across the stenosis into the periphery of the stenotic vessel.
 Alternatively, the wire is placed first, and only then is the balloon threaded over it and advanced.
Alternatively, the wire is placed first, and only then is the balloon threaded over it and advanced.
 The more distal the coronary guidewire can be placed in the vessel, the better the balloon catheter can be guided.
The more distal the coronary guidewire can be placed in the vessel, the better the balloon catheter can be guided.
Subsequently, the balloon catheter is advanced to the stenosis via the guidewire, which is held in place. With the aid of markings on the balloon catheter, it is placed so that it covers the diseased, stenotic segment completely.
 The balloon with or without stent is slowly inflated under fluoroscopy. Usually, at the beginning of the dilatation an indentation of the balloon due to the stenosis can be seen. In general, the balloon diameter is adjusted optically to the vessel diameter. The dilatation pressure is determined by the compliance of the individual balloon—depending on the manufacturer the normal diameter is usually achieved at 8 to 12 bar (higher pressures for balloons with premounted stents).
The balloon with or without stent is slowly inflated under fluoroscopy. Usually, at the beginning of the dilatation an indentation of the balloon due to the stenosis can be seen. In general, the balloon diameter is adjusted optically to the vessel diameter. The dilatation pressure is determined by the compliance of the individual balloon—depending on the manufacturer the normal diameter is usually achieved at 8 to 12 bar (higher pressures for balloons with premounted stents).
 The lower the dilatation pressure and the slower the inflation, the lower the shear forces that act on the vessel wall during POBA.
The lower the dilatation pressure and the slower the inflation, the lower the shear forces that act on the vessel wall during POBA.
 The correct location of the balloon should be verified by angiography (Fig. 22.7), especially for angioplasties near the left coronary main stem.
The correct location of the balloon should be verified by angiography (Fig. 22.7), especially for angioplasties near the left coronary main stem.
 Dilatation is done under continuous recording of the arterial pressure and of the ECG.
Dilatation is done under continuous recording of the arterial pressure and of the ECG.
 The duration of the dilatation is determined by patient tolerance, ECG changes, and the hemodynamic effects of the dilatation.
The duration of the dilatation is determined by patient tolerance, ECG changes, and the hemodynamic effects of the dilatation.
Ischemic reaction. In general, an early and marked ischemic reaction is more likely during PCI of a proximal stenosis and unimpaired ventricular function in the area supplied by the stenotic coronary artery. However, distal stenoses can also be accompanied by a severe ischemic reaction. An ischemic reaction can manifest as:
 Angina
Angina
 ST-segment depression
ST-segment depression
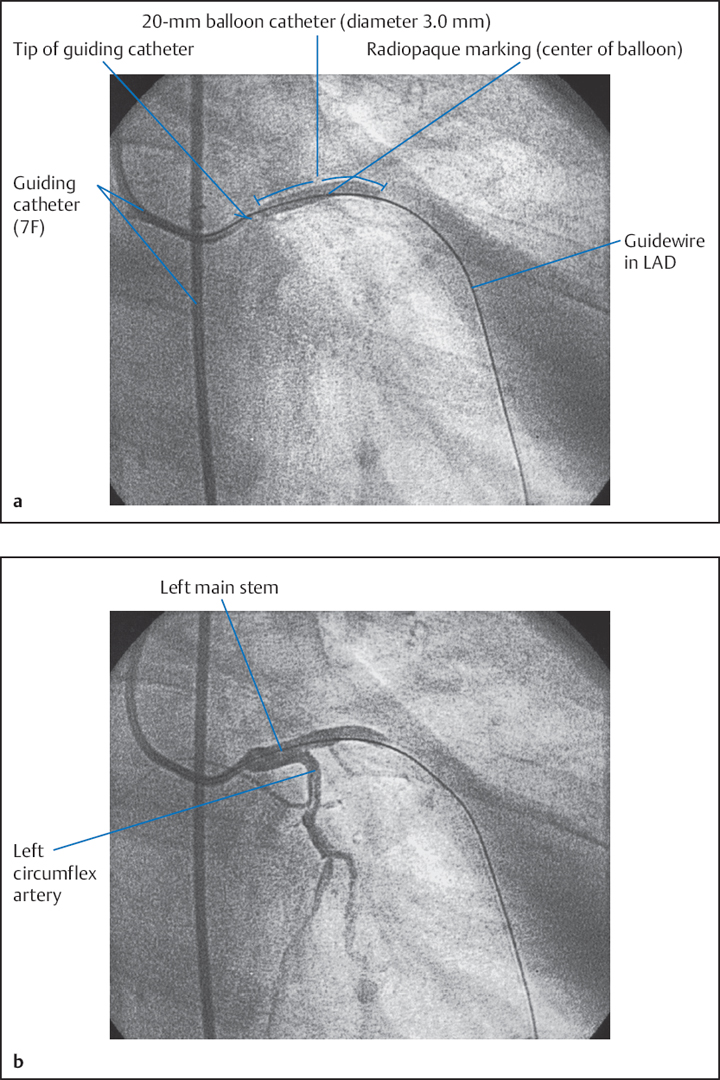
Fig. 22.7 a, b Angiographic verification of the correct balloon position for dilatation near the main stem.
a 30° RAO projection.
b 15° caudal angulation.
 ST-segment elevation
ST-segment elevation
 Ventricular tachyarrhythmias (from ventricular extra-systoles to ventricular tachycardia)
Ventricular tachyarrhythmias (from ventricular extra-systoles to ventricular tachycardia)
 Higher-degree AV block
Higher-degree AV block
 Drop in arterial blood pressure
Drop in arterial blood pressure
The individual variability in the ischemic response is substantial and ultimately cannot be accurately predicted. Some patients can tolerate dilatations up to several minutes in duration without problems, whereas others have severe complaints after only 15 seconds. Independently of this, the standard duration for dilatations can vary considerably between centers (30 seconds to 2 minutes). We usually attempt to achieve a dilatation time of 1 minute with increasing balloon pressure and thus increasing balloon diameter. This is done assuming that a somewhat longer dilatation can achieve a larger residual lumen with a lower rate of dissections (with POBA). For dilatations with DEBs, the inflation time should be at least 60 seconds
If the patient tolerates only brief dilatations, then repeated dilatations are performed with sufficiently long pauses in between (1–2 minutes). During a second inflation, patients usually tolerate longer times as a result of ischemic preconditioning.
Dilatations generally have to be kept brief (< 30 seconds) in the following situations:
 A large area is supplied by the coronary artery to be dilated (e. g., left coronary main stem, when there is a lot of “myocardium at risk”)
A large area is supplied by the coronary artery to be dilated (e. g., left coronary main stem, when there is a lot of “myocardium at risk”)
 Impaired ventricular function
Impaired ventricular function
 Contralateral akinesia
Contralateral akinesia
 Stenosis in a vessel that supplies collateral circulation
Stenosis in a vessel that supplies collateral circulation
 The last open coronary artery
The last open coronary artery
 In these patients one should not wait for a severe ischemic reaction (frequently a blood pressure drop) to occur!
In these patients one should not wait for a severe ischemic reaction (frequently a blood pressure drop) to occur!
Verification after dilatation.
 After the dilatation, repeat administration of up to 0.5 mg nitroglycerin IC
After the dilatation, repeat administration of up to 0.5 mg nitroglycerin IC
 All signs of ischemia have to disappear quickly and completely after the completion of the dilatation. If this is not the case, then the cause has to be determined (spasm, dissection, thrombus, recoil, etc.) and appropriate treatment has to be initiated.
All signs of ischemia have to disappear quickly and completely after the completion of the dilatation. If this is not the case, then the cause has to be determined (spasm, dissection, thrombus, recoil, etc.) and appropriate treatment has to be initiated.
 After completion of the dilatation the balloon catheter is withdrawn into the guiding catheter. The primary result is verified angiographically with the guidewire still in place. A residual stenosis of ≤ 20 % without significant dissection and maintained TIMI 3 flow is currently considered acceptable. Otherwise, a repeat PCI with stent implantation is performed after POBA, whereas a dilatation with a noncompliant balloon is done after coronary stenting to achieve a complete expansion.
After completion of the dilatation the balloon catheter is withdrawn into the guiding catheter. The primary result is verified angiographically with the guidewire still in place. A residual stenosis of ≤ 20 % without significant dissection and maintained TIMI 3 flow is currently considered acceptable. Otherwise, a repeat PCI with stent implantation is performed after POBA, whereas a dilatation with a noncompliant balloon is done after coronary stenting to achieve a complete expansion.
 The coronary guidewire remains in place until the final result has been achieved.
The coronary guidewire remains in place until the final result has been achieved.
 After a brief waiting period, final angiography is performed and the primary result is evaluated with the guidewire withdrawn.
After a brief waiting period, final angiography is performed and the primary result is evaluated with the guidewire withdrawn.
 If the result is good, the intervention is concluded. The puncture site is treated with a vascular closure device or, in the case of femoral access, the sheath may remain in place for later removal and compression. With radial access, the sheath is removed immediately and a pressure bandage is applied.
If the result is good, the intervention is concluded. The puncture site is treated with a vascular closure device or, in the case of femoral access, the sheath may remain in place for later removal and compression. With radial access, the sheath is removed immediately and a pressure bandage is applied.
 The patient is transferred for monitoring.
The patient is transferred for monitoring.
Procedure: Direct Stent Implantation
The aims of coronary stent-implantation are
 To reduce the risk of severe dissections, and
To reduce the risk of severe dissections, and
 To reduce the rate of restenosis
To reduce the rate of restenosis
Owing to this and to advances in stent technology, “direct stent implantation,”—that is, stent implantation without prior dilatation—has developed into the primary therapeutic procedure at many centers. This approach renders a majority of interventions simpler, faster, and safer. Numerous studies indicate that not only cost but also the rate of restenosis can be reduced.
This approach in principle is not different from balloon angioplasty. However, the following special features should be considered:
 Intervention with a 5F guiding catheter is possible for lesions with a “simple” morphology.
Intervention with a 5F guiding catheter is possible for lesions with a “simple” morphology.
 The profile is higher compared with a balloon and, therefore, the options are more limited for severe, calcified lesions.
The profile is higher compared with a balloon and, therefore, the options are more limited for severe, calcified lesions.
 Flexibility is less; therefore there is limited capability to cross highly angulated stenoses.
Flexibility is less; therefore there is limited capability to cross highly angulated stenoses.
 The rate of acute side branch occlusions is higher.
The rate of acute side branch occlusions is higher.
 The exact placement of the stent is often difficult for subtotal stenoses with lack of antegrade contrast media flow.
The exact placement of the stent is often difficult for subtotal stenoses with lack of antegrade contrast media flow.
 Special care is needed with severely calcified lesions: If the lesion cannot be dilated even with pressures > 20 bar (2 MPa), an incompletely expanded stent remains in the coronary system.
Special care is needed with severely calcified lesions: If the lesion cannot be dilated even with pressures > 20 bar (2 MPa), an incompletely expanded stent remains in the coronary system.
 The crossover rate to predilatation with a balloon is 5 to 10 %.
The crossover rate to predilatation with a balloon is 5 to 10 %.
For the operator, coronary interventions are highly demanding in terms of both skill and patient care. Not only does continuous communication between the operator and the patient reduce the fear of the patient when more severe symptoms occur during the intervention, but also it ultimately facilitates the intervention if the patient is able to cooperate well. Furthermore, accurate descriptions of current symptoms are an important criterion for assessing success.
Documentation of the Intervention
Angiographic documentation. During coronary interventions usually only the affected coronary artery or lesion is imaged in two orthogonal planes. The following ate recorded:
 Initial findings
Initial findings
 Crucial parts of the examination (correct positioning of the wire, individual balloon inflations)
Crucial parts of the examination (correct positioning of the wire, individual balloon inflations)
 Complications
Complications
 Final result (without wire)
Final result (without wire)
Protocol. In the PCI protocol, the following data are documented:
 Patient data
Patient data
 Clinical diagnosis: cardiac history in note form, current symptoms (CCS classification of angina), relevant prior interventions, and comorbidities
Clinical diagnosis: cardiac history in note form, current symptoms (CCS classification of angina), relevant prior interventions, and comorbidities
 Angiographic diagnosis and indication: crucial findings of the coronary angiography and stenosis morphology according to AHA/ACC; ischemia and viability as assessed in functional tests
Angiographic diagnosis and indication: crucial findings of the coronary angiography and stenosis morphology according to AHA/ACC; ischemia and viability as assessed in functional tests
 Materials: guiding catheter, balloon catheter, stent, guidewire
Materials: guiding catheter, balloon catheter, stent, guidewire
 Vascular access
Vascular access
 Fluoroscopy time, radiation dose
Fluoroscopy time, radiation dose
 Duration of examination
Duration of examination
 Amount of contrast medium
Amount of contrast medium
 Hemodynamics: usually aortic pressure
Hemodynamics: usually aortic pressure
 Course of the examination: detailed description of the angioplasty, including number of dilatations, duration of dilatation, dilatation pressure, equipment used (balloon, stent, protection system, etc.), ischemic reaction (ECG changes, symptoms, and hemodynamics), medication with dosage, lack or occurrence of complications, final result with quantification of the residual stenosis, and description of any dissection
Course of the examination: detailed description of the angioplasty, including number of dilatations, duration of dilatation, dilatation pressure, equipment used (balloon, stent, protection system, etc.), ischemic reaction (ECG changes, symptoms, and hemodynamics), medication with dosage, lack or occurrence of complications, final result with quantification of the residual stenosis, and description of any dissection
 Postprocedural care
Postprocedural care
Postprocedural Care
The following are appropriate for an uncomplicated course and good primary result:
 ECG after completion of the PCI
ECG after completion of the PCI
 A brief monitoring phase until the arterial sheath is removed (monitoring unit). Blood coagulation usually normalizes only after 4 to 6 hours (sheath removal if ACT < 150 seconds).
A brief monitoring phase until the arterial sheath is removed (monitoring unit). Blood coagulation usually normalizes only after 4 to 6 hours (sheath removal if ACT < 150 seconds).
 After stent implantation, if not already pretreated with clopidogrel: a loading dose of 600 mg orally
After stent implantation, if not already pretreated with clopidogrel: a loading dose of 600 mg orally
 Pressure bandage and bed rest for 12 hours
Pressure bandage and bed rest for 12 hours
 Alternatively:
Alternatively:
– Immediate removal of the sheath and primary vessel occlusion with a vascular occlusion device (e. g., Angio-Seal, Vasoseal, Starclose)
– Immediate removal of the sheath and pressure bandage for radial access
 Regular monitoring of the circulation, the puncture site, and the pulse
Regular monitoring of the circulation, the puncture site, and the pulse
The following are appropriate for high-risk patients such as those with angiographically visible thrombus, with suboptimal primary result (remaining dissection, residual stenosis > 30 %), or with acute coronary syndrome:
 Repeat resting ECG immediately after completion of the PCI
Repeat resting ECG immediately after completion of the PCI
 Monitoring for additional 24 hours
Monitoring for additional 24 hours
 Sheath removal after normalization of blood coagulation; pressure bandage and bed rest for up to 24 hours
Sheath removal after normalization of blood coagulation; pressure bandage and bed rest for up to 24 hours
 Continuation of effective anticoagulation, for example, with heparin or low-molecular-weight heparin
Continuation of effective anticoagulation, for example, with heparin or low-molecular-weight heparin
 If appropriate, a GP IIb/IIIa inhibitor IV for up to 48 hours
If appropriate, a GP IIb/IIIa inhibitor IV for up to 48 hours
 Nitroglycerin IV for 24 hours
Nitroglycerin IV for 24 hours
 Measurement of cardiac-specific biomarkers (creatine kinase MB, troponin) after 3 and after 12 to 24 hours
Measurement of cardiac-specific biomarkers (creatine kinase MB, troponin) after 3 and after 12 to 24 hours
 Problems Associated with PCI
Problems Associated with PCI
Dissections
Dissections of the coronary artery occur in ~20 to 45 % of cases of balloon angioplasty. During PCI, the adventitia and media of the vessel are extended. However, the inelastic segments of the intima and the atherosclerotic plaque tear. With further extension the tear becomes larger both circumferentially as well as longitudinally, so that the plaque is partially lifted from the vessel wall.
 The more complex the initial morphology or the stenosis, the higher the likelihood of dissection (~90 % for type C stenoses).
The more complex the initial morphology or the stenosis, the higher the likelihood of dissection (~90 % for type C stenoses).
Localized dissections with a small intimal tear are usually without sequelae, whereas extended dissections regularly result in abrupt vessel closure.
According to the National Heart, Lung, and Blood Institute (NHLBI) dissections are categorized into the following types:
 Type A: minor radiolucencies within the coronary lumen during contrast injection without dye persistence (“haziness”)
Type A: minor radiolucencies within the coronary lumen during contrast injection without dye persistence (“haziness”)
 Type B: parallel tracks or double lumen during contrast injection
Type B: parallel tracks or double lumen during contrast injection
 Type C: persistent extraluminal contrast cap (partial detachment of the plaque)
Type C: persistent extraluminal contrast cap (partial detachment of the plaque)
 Type D1: spiral filling defect with normal contrast flow (spiral dissection)
Type D1: spiral filling defect with normal contrast flow (spiral dissection)
 Type D2: spiral filling defect with delayed contrast flow
Type D2: spiral filling defect with delayed contrast flow
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Premedication
Premedication