Earlier attempts at percutaneous closure of perimembranous ventricular septal defects (Pm VSDs) were abandoned because of incidence of heart block likely as a result of device rigidity and/or oversizing. This is retrospective review and data reporting of patients who underwent percutaneous closure using the softer second-generation Amplatzer vascular occluders; namely the Amplatzer vascular plug, second generation, (AVP II) and the Amplatzer duct occluder, second generation (ADO II) in our institution. A total of 20 patients were identified; AVP II was used in 9 patients and ADO II in 11 patients. Median weight was 13.45 kg (range 6.5 to 76); age 28.5 months (range 11 to 352). After procedure, 4 were noted to have aortic insufficiency; trivial in 3 and mild in 1 (unrelated to the device). Mild tricuspid regurgitation possibly device or procedure related was seen in 4. Residual flow through the device was common after procedure and disappeared in all but 3, graded as trivial in 1, small in 2. Average follow-up period was 7.54 months ± 7.5 (1 day to 25 months). There was no incidence of heart block, bacterial endocarditis, hemolysis, device embolization, or fracture. The aortic insufficiency resolved in 1 patient and was estimated to be trivial in the remaining 3 patients. In conclusion, percutaneous closure of Pm VSDs using the softer new generation devices as the AVP II and the ADO II is feasible and safe. Longer follow-up and larger series are needed.
At the present time, there is no readily available device for percutaneous closure of perimembranous ventricular septal defects (Pm VSDs). Initial attempts at percutaneous closure of Pm VSD resulted in heart block in 3% to 5% of patients in some reports. Greater procedural complications were encountered in patients weighing <10 kg. This is likely related to device delivery requiring prograde placement of sheaths after forming arteriovenous loops in relatively small hearts. More recently, softer devices have been introduced to the market as the Amplatzer vascular plug, second generation, (AVP II) and Amplatzer duct occluder, second generation, (ADO II), (St Jude Medical/AGA Medical, Plymouth, Minnesota). In our institution, selected patients with Pm VSDs were offered percutaneous closure using these newer devices. These devices are soft and can be delivered either prograde or retrograde through relatively small catheters. This allowed easier delivery without the usual need for sheath placement in the left ventricle.
Methods
We have considered patients with Pm VSDs including those with very small separation of the aortic valve for percutaneous closure using these softer, newer devices. Informed signed consent was obtained from the family after thorough discussion about the alternative procedures, the novel use of this device and the potential risks involved with the percutaneous closure. Most (not all) families preferred the percutaneous closure. We performed retrospective review of the patients who underwent percutaneous closure of Pm VSDs in our institution using either of these devices and is the focus of this report. Angiography and transesophageal echo were used during the procedure. Using the femoral approach, the VSD was usually crossed by the aid of a retrograde advanced Judkins right type catheter, directing a soft tipped wire as a 0.014 inch ChoICE PT wire with a 35 cm floppy end (Boston Scientific, Heredia, Costa Rica), with a coaxial microcatheter as the Maestro catheter (Merit Medical Systems, South Jordan, Utah) or Cantata 2.5 (Cook, Bloomington, Indiana) or TigerWire (St Jude Medical, Minnetonka, Minnesota). The wire was snared and stabilized. If retrograde closure was performed, the Judkins right catheter was replaced with the delivery catheter and the occluder placed using that route. In cases of prograde closure, crossing of the defect was similar to what was described for retrograde closure. The wire was carefully exteriorized from the venous side ( Figure 1 ), and the delivery catheter (and subsequently the device) placed from that approach ( Figure 2 ). Modification to the previously mentioned technique was done as needed. After release, further imaging was obtained to confirm optimum placement without interference with the aortic valve ( Figure 2 ).
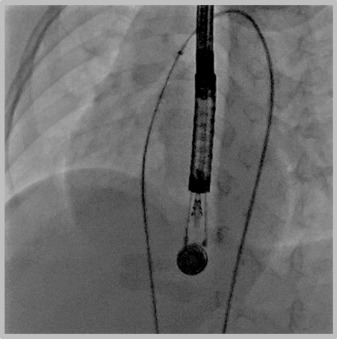
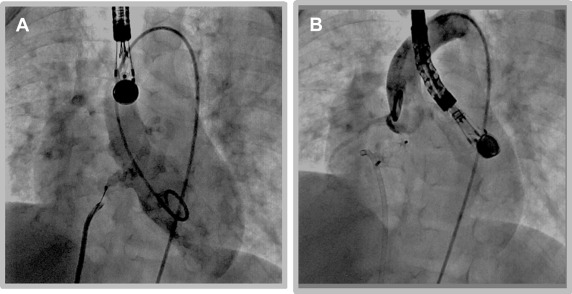
Results
From April 2010 to May 2015, 20 patients underwent percutaneous closure of Pm VSD using the AVP II or ADO II in our institution. Additional diagnosis included mild double-chamber right ventricle (not requiring surgery) in 1 patient, postoperative VSD in 3 patients (2 Tetralogy of Fallot, and 1 truncus arteriosus), history of surgical resection of double-chamber right ventricle in 1 patient, pulmonary valve stenosis in 1 patient, and atrial septal defect in 1 patient. Their median age was 28.5 months (range 11 to 352), median weight 13.45 kg (range 6.5 to 76). Ten of the devices were delivered retrograde and 10 using the prograde approach. Median fluoroscopy time was 27.2 minutes (range 8.1 to 43.8; including balloon pulmonary artery angioplasty in 2 postoperative patients, closure of an atrial septal defect in 1 patient and balloon pulmonary valvuloplasty in1 patient). Median procedure time was 111.5 minutes (range 60 to 162 minutes). All patients except 1 had VSD of considerable size on the basis of increased left ventricle size, cardiomegaly on chest x-ray, Qp:Qs >1.5:1, or evidence of congestive heart failure requiring anticongestive treatment. The 1 patient with a small VSD underwent closure because her primary obstetrician preferred to have her VSD closed before starting infertility treatment. Another patient with cardiomegaly on chest x-ray had valvar pulmonary stenosis (physiology of pulmonary artery banding) limiting the shunt calculated in the catheterization laboratory despite average size VSD by imaging. After successful balloon dilatation of the pulmonary valve, the VSD was closed in the same cardiac catheterization. The average Qp:Qs including the 2 patients with limited shunts, which were closed for the reasons discussed previously, was 1.67 ± 0.67. All the patients were discharged home the following morning, except 1 patient who was in the pediatric intensive care unit before the procedure and required truncal valve surgery unrelated to the procedure. The average distance between the VSD rim and the aortic valve leaflet attachment was 3.47± 1.9 mm (range 1 to 7 mm). When the VSD was very close to the aortic valve, but the placement was intended within the “VSD aneurysmal tissue pocket,” the distance was measured between the edges of the pocket to the aortic rim. The size of the VSD was estimated by the color width on transesophageal echo examination. When the ADO II was used, the size of the center disc was chosen to be equal to or 1 mm larger than the size of the color Doppler imaging. If AVP II was used, it was chosen to be 6 to 7 mm larger than the VSD size by color Doppler, which would be equivalent to the ADO II retention disc size. Depending on the distance to the aortic valve, and when AVP II was used, either 2 discs were on the left ventricular side or on the right ventricular side of the defect. The AVP II was used in 9 patients and ADO II in 11 patients. In 1 patient, an initially placed ADO II device embolized and was retrieved. The defect was closed in the same procedure using an AVP II with 2 mm larger retention disc. Transthoracic echo ( Figure 3 ) and electrocardiogram (ECG) were obtained in all patients. The average follow-up period was 7.54 months (±7.4) (range 1 day to 25 months). Four patients had new aortic insufficiency (AI) after procedure not related to the device position; graded as trivial in 3 and mild in 1. On most recent follow-up, the AI has resolved in 1 patient with previously trivial AI. The mild AI seen in 1 patient was central at the leaflets co-optation. It may be related to mild degree of root dilatation and/or mild leaflet dysfunction. This has improved and was estimated to be trivial in the most recent echo. There was mild new tricuspid insufficiency in 3 patients. One patient who is a 29-year-old has had only 1-month follow-up. Subsequent evaluations were with her primary physicians, and data were not available to us. The rest of the patients are being followed and are doing well. No patient had a history of hemolysis, syncope, subacute bacterial endocarditis, or cerebral vascular accidents. ECGs on most recent follow-up showed sinus rhythm in all patients except 2 patients. One of these 2 patients has bilateral superior vena cavae and had low atrial rhythm before the procedure. The ECG on follow-up showed accelerated junctional rhythm at a rate of 100 beats/min. A subsequent ECG showed low atrial rhythm (similar to precatheterization rhythm). The second patient had junctional rhythm alternating with sinus in the catheterization laboratory before placing the device. The pattern was seen after device placement but has reverted to normal sinus rhythm in most recent evaluation. Immediately, postclosure residual flow was frequently seen through the device material. This is not unexpected because the device has no occlusive material. The flow usually ceases after device endotheliolization. The residual flow disappeared in all but 3, 1 with trivial residual flow and 2 with small flow above the device (one of the latter ones had only follow-up at 1 day after procedure). None was considered clinically significant to warrant surgery. One patient with small residual VSD had a repeat cardiac catheterization, which showed normal pulmonary artery pressure and Qp:Qs ≤1.5:1 (decreased from 3.1:1).




