18 Pediatric Pacing and Defibrillator Use
Permanent cardiac pacemakers have been used in children for 60 years.1 Technologic advances have increased pediatric use through customized pacemaker design and a smaller, longer-lasting generator. Although many aspects of pediatric pacing are similar to adult pacing, children are not only physically smaller than adults, but also have different underlying cardiac diseases. Life circumstances differ, and pediatric patients face longer lifetime therapy. Therefore, differences exist not only in selection of the optimal pacing system, but also in implantation techniques, programming considerations, and follow-up methods.
 Midwest Pediatric Pacemaker Registry
Midwest Pediatric Pacemaker Registry
Because the number of patients with congenital heart disease requiring pacemakers is small, a large study at one center is lacking. Therefore, conclusions are based on limited experience susceptible to statistical inaccuracies. To address this problem, the Midwest Pediatric Cardiology Society formed the Midwest Pediatric Pacemaker Registry (MPPR) in 1980. Member institutions submit data on patient demographics, pacing indication, associated structural cardiac disease, type of generator/electrode and threshold data at implantation, and device explantation data (Table 18-1). No long-term follow-up data are provided. Annual reports are presented to promote data submission and validity, address concerns about the types of data collected and the methods used, and ensure uniformity among participating institutions.
TABLE 18-1 Midwest Pediatric Pacemaker Registry: Data Collection*
| Information | Data Collected |
|---|---|
| Patient | |
| Generator | |
| Electrode |
RMS, Mean spontaneous waveform amplitude.
* Data are collected on all new patients entered in the Registry, all generators implanted and explanted, and all electrodes implanted, explanted, or invasively tested.
 Indications for Permanent Pacemaker Implantation
Indications for Permanent Pacemaker Implantation
Sinus Node Dysfunction
Most of these patients have undergone cardiac surgery many years earlier, usually involving extensive atrial procedures. The most common procedure is the atrial switch operation for transposition of the great arteries.2 The likelihood of these patients needing permanent pacing increases with time since surgery.3 Even though the use of this procedure for simple dextrotransposition of the great arteries is now rare, its use is increasing in more complex disease, such as levotransposition of the great arteries combined with the arterial switch procedure (“double switch”) to place the morphologic right ventricle in the pulmonary circuit and the morphologic left ventricle in the systemic circuit.
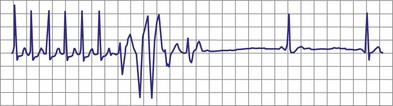
With increased use of the Fontan procedure, or right-sided heart bypass, the incidence of sinus node dysfunction is increasing. The indications are similar to those for congenital complete heart block. In addition, the presence of tachyarrhythmias, with the subsequent risk for prolonged asystole after acute termination of the tachycardia, is also an indication for pacemaker implantation (Fig. 18-1). In patients with sinus node dysfunction after cardiac surgery, our practice is to recommend pacemaker implantation in all patients with a sleeping heart rate of less than 30 beats per minute (bpm) even in the absence of symptoms, a decreasing exercise tolerance with inadequate heart rate increase with exercise (see later discussion), or sinus pauses for longer than 3 to 4 seconds. The need for medications known to affect atrioventricular (AV) conduction for the control of tachyarrhythmias in these patients would also necessitate pacemaker placement.3
Surgically Induced Heart Block
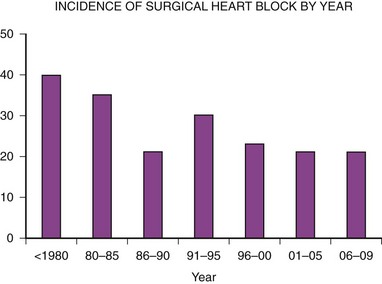
The second major indication for pacemaker implantation in children is surgically induced heart block, which classically has accounted for 30% to 40% of children undergoing pacemaker implantation.4–9 MPPR data show that the indication for initial pacemaker placement was surgically induced heart block in an average of 35% of patients before 2000 (Fig. 18-2). The percentage varies from year to year and reached a low of 20% in 2001-2005. There has been no definite downward trend since 2000, with the percentage remaining near 20%, although the underlying structural cardiac diseases in patients with surgically induced heart block have changed dramatically. Since 2000, surgical heart block has accounted for 15% to 25% of initial implantations. Most recent data from our center show that the incidence is 22% over the last 5 years. Most children acquiring surgical heart block in the last 5 years have had complex disease and have undergone complex surgical repairs. The surgical procedure resulting in the greatest incidence of heart block is the repair of atrioventricular septal defect, which has accounted for 17% of patients with surgical heart block since 1988. Table 18-2 lists the other common diagnoses associated with surgical heart block in the recent era.
TABLE 18-2 Most Prevalent Structural Cardiac Defects Associated with Surgically Induced Complete Heart Block*
| Defect | Percentage of Cases |
|---|---|
| Atrioventricular septal defects | 17 |
| Isolated ventricular septal defect | 14 |
| Dextrotransposition of the great arteries | 12 |
| Levotransposition of the great arteries | 12 |
| Tetralogy of Fallot | 7 |
| Aortic valve replacement | 3 |
* The most common structural cardiac lesions associated with surgically induced heart block at the time of complete repair for children undergoing initial implantation since 1988.
Data from Midwest Pediatric Pacemaker Registry.
Surgical heart block can develop at the initial cardiac repair or later. In addition, the heart block acquired at repair may be temporary, with return of reliable AV conduction. For this reason, our current practice is to implant only temporary pacing electrodes at the initial surgery and to defer permanent pacemaker implantation for 10 to 14 days in the hope of a return of AV conduction. However, ventricular escape rhythms are unstable, and no child with a permanent, surgically acquired complete heart block is discharged without a permanent pacemaker. Even in the hospital, all children are supported with an external pacemaker through temporary pacing wires placed at surgery until consistent AV conduction returns or a permanent pacemaker is inserted. Monitoring should consist of both electrocardiographic (ECG) monitoring and non-ECG monitoring, such as arterial pressure measurements or pulse oximetry. Many ECG monitors detect the pacing artifact and do not recognize the lack of capture with subsequent bradycardia or asystole. This is avoided by the use of a non-ECG method of detecting cardiac ejection, such as pulse oximetry.10
Congenital Complete Heart Block
The next most common indication for pacemaker implantation is congenital complete heart block. The cause of congenital heart block varies; an autoimmune mechanism is often implicated, with clinical or laboratory evidence of connective tissue disease in the mother.11 All mothers of infants with congenital heart block should have antibody determinations performed. Even children born to such mothers but with intact conduction at birth must be monitored for the development of heart block over time. Treatment of antibody-positive mothers with steroids during pregnancy has not been shown to alter outcomes or reverse the development of heart block. Congenital heart block is also associated with specific forms of structural disease, particularly those involving abnormalities of the AV junction, such as levotransposition of the great arteries with AV discordance and atrial situs ambiguus.12 It is common for fetal heart block to “develop” in utero with intact conduction present in the young fetus and heart block developing at 20 to 30 weeks of gestation.
Data from the MPPR indicate that 10% to 25% of patients have congenital heart block as the primary indication for permanent pacing. Most recent data from our center show that 17% of patients receiving pacemakers have congenital heart block. The age at which the pacing system is implanted varies, ranging from a few hours to more than 20 years. Most children with associated structural cardiac disease who need pacing before 1 year of age have congestive heart failure (CHF) requiring an increased heart rate for adequate therapy. The mortality rate in such children is also high, with 43% dying by 2 years of age.13
For children with structurally normal hearts and congenital heart block, the incidence of pacemaker implantation is lower in younger children but increases with age, associated with a gradually decreasing ventricular rate.14 The gradual and steady increase in the need for permanent pacing continues with advancing age, reaching 75% by age 20 years (Fig. 18-3). The need for permanent pacing results from the development of syncope, CHF, or increasing ventricular ectopy, often associated with prolongation of the corrected QT interval (QTc). Death is rare in children with no structural cardiac disease (only 5% by age 20) but can occur suddenly.
Current recommendations call for the implantation of a permanent pacemaker system whenever CHF is present. In addition, implantation is recommended if the average heart rate is less than 50 bpm in the awake child and 55 bpm in the infant, if there is a history of a syncopal or presyncopal event, if significant ventricular ectopy is present, or if there is exercise intolerance.15,16 However, symptoms of exercise intolerance can be difficult to elicit. Many children deny such symptoms, as do their parents, when in fact their exercise tolerance would be improved with permanent pacing. Many parents return after pacemaker implantation to relate that the activity level of their child has greatly increased. They are amazed at this change, because they did not believe that the child was significantly hindered before pacemaker implantation. Exercise testing is often useful as an indicator of the child’s exercise capabilities compared with those of a normal child. The physician should also periodically assess the child for increasing cardiac size by chest radiography and for decreasing cardiac function by echocardiography. The presence of either of these conditions should be considered an indication for permanent pacemaker placement.
Some children with congenital complete heart block develop a tachydysrhythmia, specifically ventricular tachycardia (VT), which can be controlled only with permanent pacing.17 The maintenance of a minimal heart rate often suppresses the tendency toward ventricular ectopy, particularly during exercise. The development of tachyarrhythmias with the stress of exercise, even in children with otherwise asymptomatic disease, necessitates pacemaker implantation.
Other Indications
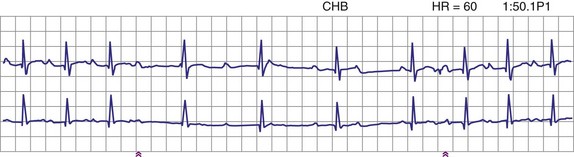
Patients with long QT syndrome and uncontrollable VT may also benefit from pacemaker placement, as well as those with intermittent complete heart block18 (Fig. 18-4). A chronic increase in heart rate shortens the QT interval and decreases the occurrence of VT. The combined use of pacing and an ICD may be even more efficacious, particularly with the advent of the dual-chamber ICD.
Other indications for pacemaker placement reported in the MPPR include the need for control of atrial tachyarrhythmias unresponsive to pharmacologic therapy, second-degree heart block associated with symptoms, and concern about a sudden loss of AV conduction in patients receiving certain antiarrhythmic therapies known to interfere with AV conduction. Although such indications are rare, the clinician should not restrict pacemaker use to those children with complete heart block. First-degree heart block and trifascicular block with no documented loss of AV conduction are not considered indications for pacemaker implantation.19
A relatively controversial indication for pacemaker placement is symptomatic hypertrophic obstructive cardiomyopathy with significant outflow tract obstruction. Although pacemaker placement is not effective in all children with this disorder, both hemodynamic and symptomatic improvements have been observed,20 with decreases in gradient and measures of diastolic performance. Generators used for this indication must allow programming of relatively short AV intervals and rate-adaptive AV intervals to maximize the QRS width and degree of preexcitation. Younger patients with more rapid heart rates may present insurmountable difficulties, and other therapies are probably indicated initially. When pacing is employed in this setting a dual-chamber ICD should be used (see later).
 Selection of the Appropriate Pacemaker System
Selection of the Appropriate Pacemaker System
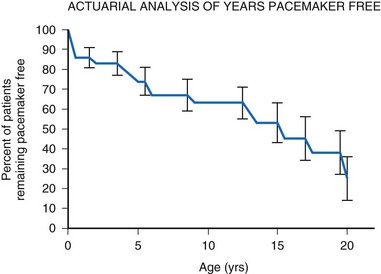
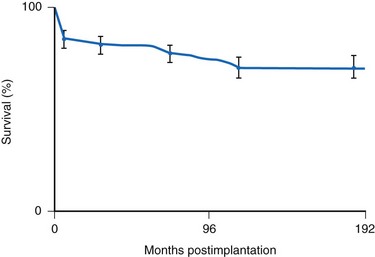
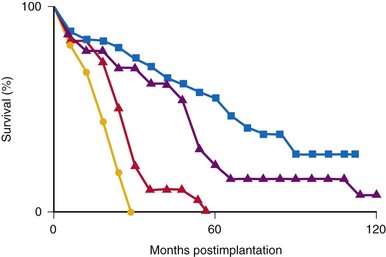
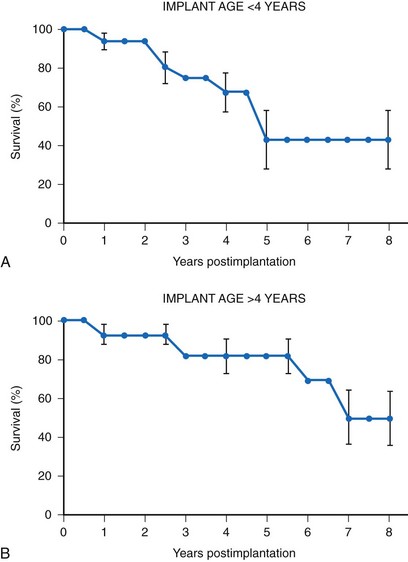
Many factors must be considered in the selection of the most appropriate pacemaker generator and electrode system. Unlike that in the adult patient, the 5-year patient survival rate after pacemaker implantation in children exceeds 70% (Fig. 18-5), and death is usually related to the underlying structural heart defect.8,21 Therefore, pacing may be needed for more than 50 years in the average child. This affects pacing choices, because the number of replacement generators and electrodes may be high. The average longevity of currently available pulse generators is only 5 years when all children are grouped together (Fig. 18-6). However, when children are divided into those younger and those older than 4 years at generator implantation, longevity is much different (Fig. 18-7). The generator half-life is 5 years for children younger than 4 at implantation and increases to 7 years for the older children. This is presumably a result of the higher heart rates present in younger patients, when the device is used in dual-chamber mode to track the atrial rate, and the higher programmed lower rates used in younger children. Initially, epicardial electrodes used in the younger children contributed to higher current drain. With newer epicardial electrodes, this difference has largely disappeared.
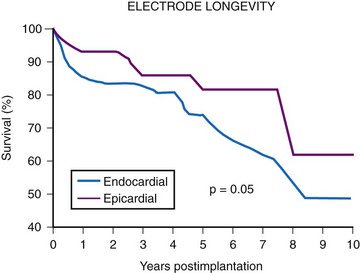
The average epicardial electrode lasts 7 years.22 With improvements in epicardial electrode design, it is hoped that this will increase. Although the average endocardial electrode’s longevity in children is significantly increased, it is still only slightly more than 10 years23 (Fig. 18-8). For the child undergoing an initial implantation at age 1 year, a minimum of nine electrode changes and 17 generator changes can be expected. The multiple procedures that will be needed and the effects of one on subsequent procedures must be considered.
Generator Mode Selection
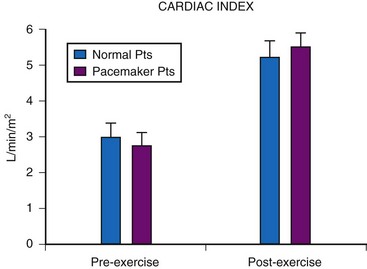
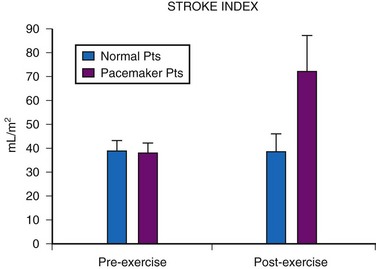
Although cardiac output increases with exercise, even during fixed-rate pacing (Fig. 18-9), this results from a large increase in stroke volume (Fig. 18-10), with presumed increased wall stress and potentially increased myocardial work compared with the same change in cardiac output, when a heart rate increase is possible.24 However, enhanced exercise tolerance is achieved when rate-variable pacing is used.25 This suggests an advantage to rate-variable pacing in the child who is expected to lead an active life. The rate-responsive mode should always be used unless the patient can demonstrate an adequate intrinsic rate response to exercise by exercise testing or ambulatory electrocardiogram.
Single-chamber pacing in either the atrium or the ventricle has been advocated for the treatment of sick sinus syndrome.15 When atrial pacing is chosen, the presence of normal AV node function must be established by provocative electrophysiologic testing before implantation, especially in the postsurgical patient, because AV nodal disease can accompany SA nodal disease and may not be apparent in the resting, nonprovoked state. AAI(R) pacing has the advantage of preserving the normal ventricular activation sequence with potentially better cardiovascular function. In addition, some evidence in animals points toward the long-term development of myocardial changes when an abnormal pattern of myocardial activation is present.26 A comparison of cardiac myocyte changes in ventricular free wall pacing versus high septal pacing near the bundle of His, which has a narrower QRS morphology, is striking; the clinical implication of these changes is unknown.27
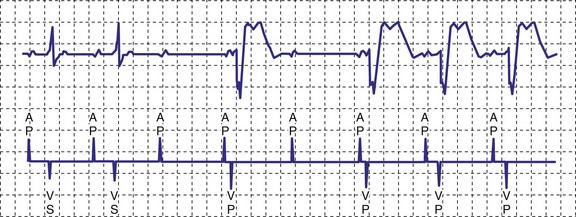
A newer pacing mode, managed ventricular pacing (MVP; Medtronic) has great utility in a patient with predominantly sinus node dysfunction but also with intermittent lack of AV conduction.28 In this mode, pacing is AAIR, but the device monitors for ventricular activity between two consecutive P waves without regard to timing. If two consecutive P waves occur without an intervening R wave, a ventricular pace is generated. If this occurs twice within four P waves, the device shifts mode to DDD(R). After a predetermined time, the ventricular output is inhibited, and the device searches for an R wave between two P waves. If one occurs, the mode reverts to AAIR; if not, DDDR mode remains in force (Fig. 18-11).
Single-chamber ventricular pacing, or VVI(R), allows the use of more stable electrode systems and, in the rate-responsive mode, still allows rate variability to be maintained in the ambulatory child. The importance of atrial systole in the maintenance of cardiac output is debatable and varies from child to child. Because most children have good myocardial function, the atrial contribution is probably minimal. The cardiac output increase with exercise is improved in children with DDD versus VVI pacing. It is unclear, however, whether this increase is the result of atrial synchrony or rate variability. Pacemaker syndrome from VVIR pacing is uncommon but can occur, especially over time. In one series, 19 of 33 patients developed symptoms suggestive of pacemaker syndrome over time (median, 11 years) that resolved with upgrading to dual-chamber pacing.29 The major factor to be considered in such a choice is the difficulty in placing an adequate atrial electrode. If prior surgery or underlying structural disease precludes atrial electrode placement, single-chamber pacing is an acceptable alternative. However, dual-chamber pacing should be considered for all patients, with single-chamber pacing used only if contraindications to dual-chamber pacing exist, as discussed later. Even in patients with sinus node dysfunction, dual-chamber pacing should be considered, particularly if AV nodal function is suspect.
We now consider dual-chamber pacing to be the mode of choice in children. We use single-chamber pacing only if a contraindication to dual-chamber pacing exists, as well as in some small infants who will need cardiac surgery in the immediate future, with an upgrade to dual-chamber pacing performed at that time. The major contraindications to dual-chamber pacing are (1) persistent atrial tachyarrhythmias; (2) changing AV nodal status, making numerous programming changes necessary; and (3) inability to place reliable atrial and ventricular electrodes. An example of the third contraindication is the small child in whom endocardial pacing is preferred, but the presence of two electrodes in the superior vena cava might present a high risk for thrombosis. Another example is the child requiring epicardial electrode placement in whom atrial electrode placement would necessitate a greatly enhanced surgical procedure. One must remember that rate sensors do not function in nonambulatory infants. Therefore, in young infants, single-chamber pacing becomes fixed-rate pacing. The generator’s size and functionality are no longer considerations, because dual- and single-chamber pacemakers are comparable in both regards. Table 18-3 lists the most common contraindications to dual-chamber pacing.
TABLE 18-3 Major Contraindications to Dual-Chamber Pacing in Children
| Contraindication | Causes |
|---|---|
| Inability to place both an atrial and a ventricular electrode | |
| Persistent atrial tachyarrhythmia |
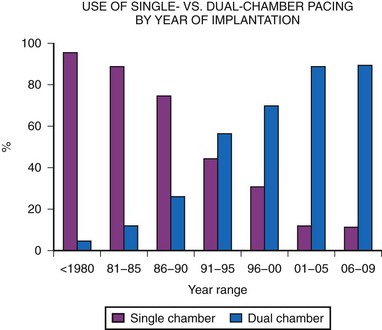
Dual-chamber pacing in children has previously been underused. MPPR data show a significant increase in dual-chamber pacing, with 43% of generators implanted in 1991 to 1992 using the DDD or DDDR mode, increasing to a majority (68%) of implantations by 1995 to 1996, and with a further increase, to more than 80%, since 2000. This is in marked contrast to the years before 1985, when fewer than 10% of generators were in the DDD mode (Fig. 18-12). This increase in dual-chamber pacing has been the result of improvements in atrial epicardial electrodes, increased experience with endocardial pacing in children, the smaller size of dual-chamber generators, and a better understanding of the benefits of dual-chamber pacing.
Generator Features
General Characteristics
The most important consideration is related to the range of energy output available, which includes both the pulse width and the pulse amplitude programmability. Although most pacemakers are programmed to have 2.5 or 5 V of amplitude, the presence of other amplitudes is of key importance. Specifically, when a generator is used with an epicardial electrode, high-output features are mandatory. Although few children require long-term pacing at output greater than 5 V, many have an initial threshold rise and temporarily require such high output. Even with endocardial implants, acute increases in threshold can occur, and the ability to increase the pacemaker amplitude to values greater than 5 V may avert the necessity for emergency electrode replacement. In addition, threshold testing at multiple low-pulse amplitudes allows a more accurate determination of the characteristics of the strength-duration curve. This testing is mandatory to determine the lowest, but still safe, pulse amplitude and width settings. The strength-duration curve characteristics are not constant, varying not only with time but also in relation to activity and time of day.30 Such changes are discussed more fully in the consideration of appropriate follow-up. Knowing where the steep part of the strength-duration curve begins is crucial for appropriate programming; the clinician wants to ensure an adequate safety margin while minimizing the energy output, to maximize generator longevity. The ability to determine thresholds at a multitude of pulse amplitudes is a necessity.
Newer pacemakers now can automatically determine the voltage threshold, either on a beat-to-beat basis or at predetermined intervals throughout the day, and then adjust the pulse amplitude within a predetermined range to minimize energy drain and potentially increase generator longevity. This is accomplished by looking for an evoked potential after the test pulse, within a predetermined window of time indicating myocardial depolarization. Initially, these pacemakers required special low-polarization electrodes to distinguish true evoked potentials from electrode polarization. Newer designs have improved this discrimination, and now this feature has been expanded to function with most types of electrodes. Some devices still require bipolar electrode systems; some do not. Both endocardial electrodes31 and epicardial electrodes32,33 have been employed with newer devices. Once the voltage threshold has been determined, the amplitude is adjusted to a predetermined value above the threshold. Threshold data are saved in the pacemaker, and later interrogation can provide the clinician with the threshold trend over time. Such a feature has been extended to the atrium as well as the ventricle in some devices. Even if the clinician is reticent to allow automatic changes to the output parameters, use of the feature in a “monitor only” mode can provide information as to the long-term changes in threshold and the potential need for programming changes. This feature has the potential to increase generator longevity and decrease the number of pacemaker replacements needed.
Another parameter often overlooked is the refractory period. In single-chamber pacing, this is often fixed to an arbitrary value of 325 msec, without much thought about whether this value is appropriate. For the ventricular channel, the refractory period must be of sufficient duration to prevent inappropriate T-wave sensing but not prevent sensing of spontaneous ventricular depolarizations. Measurement of the pace or sensing point to well beyond the T wave using the intracardiac electrogram (EGM) is straightforward (Fig. 18-13). In healthy children, the QT interval decreases with an increasing heart rate. When rate-variable pacing is used, the ventricular refractory period may be appropriate at rest but too long during exercise. Ideally, the period should vary with the pacing rate. Therefore, this value must be long enough to prevent T-wave sensing at the resting heart rate and short enough not to limit appropriate sensing at the upper pacing rate. Children with complete heart block may have spontaneous ventricular beats during the stress of exercise, which must be appropriately sensed. Appropriate programming is discussed later. Again, the wider the range of available refractory periods, the more universally applicable the pacemaker generator is to the entire pediatric population.
For AAIR pacing, the refractory period must be long enough to prevent sensing of ventricular events, but again, must not be too limiting in terms of the upper rate. Recording of the intracardiac EGM shows the extent to which ventricular events are sensed by the pacemaker and the minimum value to which the atrial refractory period may be safely programmed (Fig. 18-14).
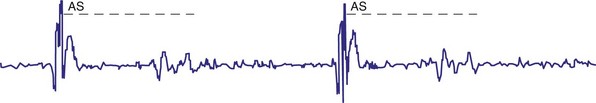
Figure 18-14 Intracardiac atrial electrogram recorded from a patient with AAI pacing. The refractory period (“dashed” line) must be long enough to prevent sensing of ventricular events by the atrial sensing (AS) electrode. Note the marked increase in refractory period required compared with VVI pacing (see Fig. 18-13).
Rate-Responsive Pacing
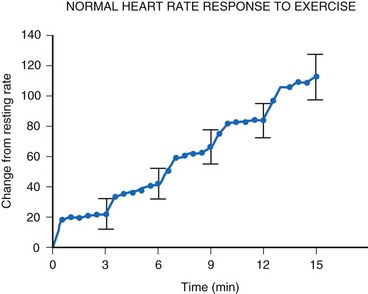
For the single-chamber rate-responsive pacemaker, appropriate settings to mimic the pediatric response to exercise are mandatory. During exercise, the healthy child’s heart rate increases linearly with the increasing intensity of the exercise34 (Fig. 18-15). When healthy children are exercised using the Bruce treadmill protocol, the heart rate increases an average of 20 bpm with each increase in exercise stage. This continues throughout the course of the exercise. After an abrupt increase in exercise intensity (from stage I to II), the heart rate shows a sudden rapid increase, reaching a plateau value. The child’s pacemaker should increase its rate in an appropriate manner with increasing exercise intensity—and this must occur quickly, reaching a plateau value rapidly for that level of intensity. Therefore, not only must the heart rate increase to an appropriate degree, but also must increase in an appropriate time frame to mimic the normal physiologic response to exercise in the pediatric patient.
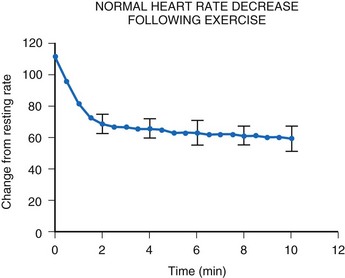
After termination of exercise, the heart rate decreases exponentially (Fig. 18-16). Although an initial rapid drop occurs, the heart rate does not reach resting levels for at least 10 minutes. Inappropriate rapid declines in heart rate after exercise termination may not meet the body’s metabolic demands and may result in inadequate cardiac output and a syncopal episode.
Numerous types of sensors have been used, the most common being activity (as a function of body vibration), blood temperature, and minute ventilation. Body vibration sensing is the most useful in children because it does not require special electrodes and is much the same in the child as in the adult. Blood temperature and minute-ventilation sensing35 have been used in children, but to a lesser degree.
Summary
Traditional factors in pediatric pacing, such as generator longevity and size, are now less important in the selection of a generator. All generators are much smaller than previous models, and yet longevity has not been sacrificed, a result of improved circuit efficiency. The difference in size among single-chamber, dual-chamber, and rate-responsive pacemakers is often undetectable. Pediatric patients generally have long life expectancy, and the ability to have a highly programmable pacemaker implanted to meet changing metabolic demands of growth, age, or patient choices is indispensable. The difference in cost between a highly programmable unit and one with fewer features is minimal, particularly when the cost is spread over the life of the pacemaker. Box 18-1 summarizes the features that must be considered for the appropriate selection of a generator. The choice of a pacemaker should be based solely on the features it possesses and its ability to meet the demands of the patient.
Pacing Electrode Selection
Endocardial versus Epicardial Pacing
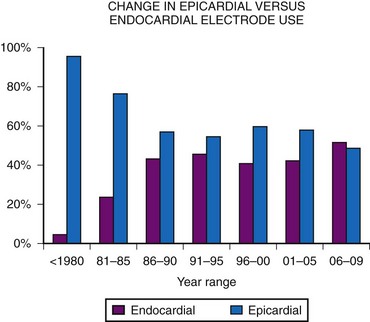
Initially, almost all electrode systems implanted in children were epicardial, because of the large size of the endocardial electrodes and pacing generators. The development of smaller electrodes and generators has changed this, although children still undergo epicardial lead placement as a result of small patient size or other factors that do not allow placement of an endocardial electrode system. MPPR data show a gradual increase in endocardial electrode use, but almost one half of all patients still receive epicardial electrodes (Fig. 18-17). Our basic approach is to assume that all children should undergo the placement of an endocardial system; we then evaluate the child for factors that do not allow endocardial electrode use. The major factors to be considered, in addition to patient size, are (1) venous access to the ventricle, (2) presence of intracardiac right-to-left shunting, (3) increased pulmonary vascular resistance, (4) right-sided prosthetic valves, and (5) severe right ventricular (RV) dysfunction or fibrosis causing either increased risk of thrombus formation or an inability to stimulate the endocardium.
Initially, it was believed that children weighing less than 15 kg (33 lb) and those younger than 4 years should not undergo placement of endocardial electrodes.36 This was based on the belief that the subclavian vein and superior vena cava (SVC) were too small, leading to a high risk for thrombosis with vessel occlusion, and that the large size of the generators made implantation in the subclavicular area impractical. With increased experience and smaller generator and electrode sizes, however, many centers now routinely implant endocardial lead systems in children weighing less than 15 kg.9,37–39 The lower range for weight is not yet known. From a technical standpoint, children as small as 3 kg (6.6 lb) can undergo endocardial electrode placement, but the follow-up of such children is too limited to know whether this is in their best interest. A study of 39 patients weighing 2.3 to 10 kg showed that 23% had some pacing system problem related to endocardial electrode placement.39 Such problems included skin necrosis over the generator, subclavian vein thrombosis, and endocarditis on the electrode. Another 23% required electrode extraction. All except one patient had received a single-chamber device.
The risk for vessel thrombosis appears to be less than once believed, at least in the short term.40 Although SVC thrombosis has been reported,41,42 the true incidence is unknown, because noninvasive methods of detecting thrombosis are not sensitive and angiography is not routinely done unless thrombosis is suspected clinically (Fig. 18-18). Lead displacement secondary to growth remains a concern, although techniques have been proposed to deal with this problem.9,38 The placement of large electrode loops within the atrium was proposed to allow for growth, but these loops may not be as effective as originally believed because they can fibrose to the cardiac wall, precluding uncoiling with growth.
The major objection to endocardial electrode use in the small child is related to long-term problems. Because young children can be expected to require numerous electrodes over their lifetime, many more than in adult patients, the clinician must consider how many electrodes can be left in place before problems with vessel obstruction or tricuspid valve dysfunction occur, as well as the difficulty of extraction of old electrodes. Although now more widely used,43,44 lead extraction still represents a significant problem in children, with the potential for damage to the cardiac structures, mainly the tricuspid valve. To commit a child to potentially numerous lead extractions is still a concern. In our institution, current guidelines call for the placement of dual-chamber endocardial systems in children weighing 15 kg or more and the placement of single-chamber endocardial systems, if single-chamber pacing is appropriate, in children heavier than 8 kg (17.5 lb). These guidelines may change as electrode development continues and as data on the long-term follow-up of children with endocardially placed electrodes become available.
The next factor that must be considered is the presence of intracardiac shunting. Electrodes are potential sources of small particulate matter, with the risk for subsequent embolization until endothelialization occurs.45 This does not tend to be a problem when particulate matter goes only to the lungs, where it is filtered out of the circulation and eventually absorbed, except in the presence of preexisting elevated pulmonary vascular resistance (PVR) or after a Fontan procedure. In the presence of right-to-left shunting, however, the potential for systemic embolization is great. The general recommendation is to avoid such electrodes in patients with documented right-to-left shunting.36 This also must be considered in patients with the potential for right-to-left shunting, even if their net intracardiac shunt is left to right. Children with atrial and ventricular septal defects can show right-to-left shunting in the setting of elevated RV pressure, even with a net left-to-right shunt.46,47 The specific hemodynamic situation of the individual child must be considered before endocardial electrode implantation is performed.
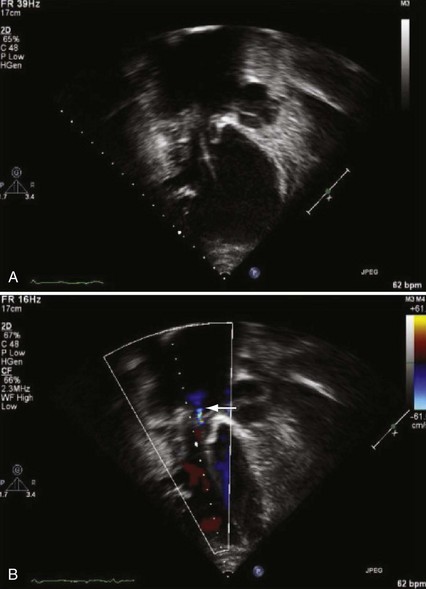
The presence of a mechanical tricuspid valve prosthesis negates the ability to use an endocardial pacing system. There have been isolated reports of endocardial electrode placements at open-heart surgery through the perivalvular area.48 This requires cardiopulmonary bypass and can be done only at valve placement. This technique cannot be used in the usual transvenous implantation; and it prevents lead extraction should that become necessary, except during repeat open-heart surgery. In cases of a heterograft valve rather than a mechanical one, the new, smaller transvenous leads have been placed without interfering with valve function (Fig. 18-19). Placement of a ventricular lead through the coronary sinus in the patient with an artifical tricuspid valve has been reported.49
The patient who has undergone a Fontan procedure presents a somewhat unique situation. As mentioned previously, controversy surrounds the best approach for electrode placement in these children. If ventricular pacing is required, the epicardial approach is the only one available. However, many of these patients have intact AV conduction, require pacing only for sinus node dysfunction, and are best served by atrial-only pacing. The original Fontan procedure connected the right atrial appendage to the pulmonary artery, leaving the entire right atrial chamber available for electrode placement. The more common techniques at present either create a tunnel along the lateral right atrial wall from the inferior vena cava to the pulmonary artery or use an extracardiac prosthetic conduit. Often, a communication exists from the system to the pulmonary atrial chamber, resulting in a right-to-left shunt. Transvenous pacing has been performed in such patients despite anticoagulation,50 but electrode placement can be difficult because of the small-system venous chamber, and right-to-left embolization with neurologic sequelae has been reported.50 For these reasons, our approach has been to avoid the transvenous approach unless the epicardial approach is not possible because of extensive fibrosis. Pacing in this group of patients can be extremely challenging regardless of the approach chosen.
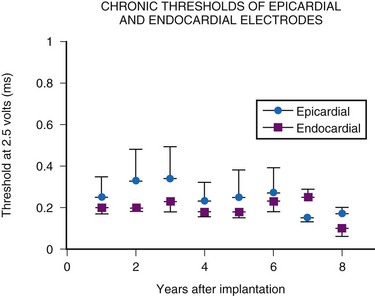
In summary, endocardial pacing is generally preferable because of the ease of implantation and the improved longevity of the electrode. Long-term thresholds are as stable as epicardial electrodes and tend to be lower (Fig. 18-20). This permits lower-output programming of the pacing generator, enhancing its longevity. However, endocardial electrode use is contraindicated in many situations (Box 18-2). As such, epicardial electrodes still play a significant role in pediatric pacing.