LEARNING OBJECTIVES
Learning Objectives
The student will be able to describe the normal physiology of pleural fluid formation and reabsorption.
The student will be to explain the diverse pathophysiological mechanisms responsible for development of pleural effusions.
The student will be able to define the categorization of parapneumonic pleural effusions and the stages of pleural space infections.
The student will be able to recognize the common symptoms and signs of pleural diseases, and the general principles of their treatment.
Diseases of the pleural space are common in clinical medicine. One of the most frequent manifestations of pleural disease is a pleural effusion. Consequently, knowledge of the pathophysiological basis for disorders that culminate in pleural effusions is important for their timely diagnosis and proper treatment. Additional information regarding the laboratory evaluation of various types of pleural effusions is presented in Chap. 19.
PHYSIOLOGY OF THE PLEURAL SPACE
The pleural space is a low-pressure environment between the parietal pleura that covers the inner surface of the ribs and thoracic musculature, and the visceral pleura covering the lungs’ external regions. These have a combined surface area of about 4,000 cm2. The forces regulating pleural fluid formation by the parietal and visceral pleura are summarized by the Starling’s equation (as established in Chaps. 7 and 19):
Notably, the protein reflection coefficient σ defines the barrier function of the pleural membranes, such that decreases in σ typified by inflammatory conditions correlate with increased permeability to protein (see also Chap. 28). The intravascular concentration of albumin (MW ≈ 65 KDa) is the primary determinant of serum colloid osmotic pressure.
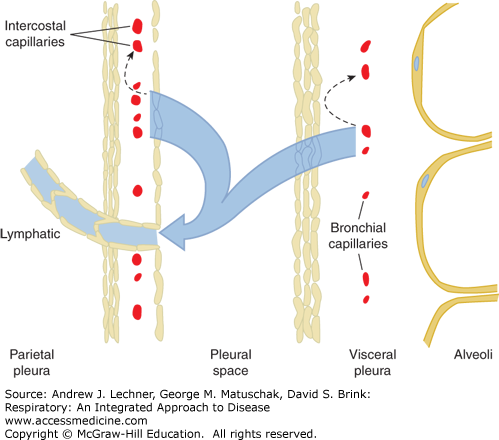
The normal entry rate of pleural fluid into the pleural space in humans is considered to be approximately 0.5 mL/h or 12 mL/day, and this pleural fluid derives from microvascular filtration governed by Starling’s forces across both the parietal pleura and visceral pleura. Although a degree of fluid reabsorption occurs within both the parietal and visceral pleura membranes, actual pleural fluid reabsorption from the pleural space itself occurs via lymphatic stomata in the parietal pleura (Fig. 29.1). Thus, at any time the total volume of pleural fluid in normal individuals is 0.1-0.2 mL/kg, or approximately 10-20 mL in a 70 kg subject. These parietal pleural lymphatic stomata are fenestrated openings in the mesothelial cell layer that average 10-12 μm in diameter, and are especially prevalent in dependent regions of the pleural space, especially on the diaphragmatic surface and in the mediastinal regions (Fig. 29.2).
FIGURE 29.1
Representation of normal pleural fluid formation and reabsorption. Microvascular filtrate from microvessels in the parietal and visceral pleura is partly reabsorbed within each membrane (dashed arrows), whereas the remaining low-protein interstitial fluid traverses the pleural mesothelia by bulk flow into the pleural space. Pleural fluid exits the pleural space via lymphatic stomata in the parietal pleura. Redrawn from Chretien et al. The Pleura in Health and Disease. Marcel Dekker; 1985.
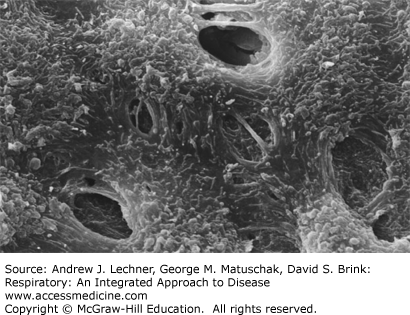
FIGURE 29.2
Scanning electron micrograph of monkey parietal pleura demonstrating lymphatic stomata interposed between the smaller cuboidal mesothelial cells. From Miura T et al: Lymphatic drainage of carbon particles injected into the pleural cavity of the monkey, as studied by video-assisted thoracoscopy and electron microscopy, J Thorac Cardiovasc surg Sep; 120(3):437-447, 2000.
PATHOPHYSIOLOGY OF PLEURAL EFFUSIONS
There are seven general mechanisms by which pleural effusions can develop (Table 29.1). Students should be aware that these can occur singly or in combination.
| Mechanism | Clinical Examples |
|---|---|
| Increased Pmv in parietal pleural microvessels | Congestive heart failure |
| Decreased πmv in blood | Cirrhosis, nephrotic syndrome |
| Increased permeability of pleural microvessels | Inflammation, infection, tumor |
| Decreased lymphatic drainage | Lymphoma, yellow nail syndrome |
| Decreased peri-microvascular pressure | Atelectasis, trapped lung |
| Transdiaphragmatic flow of peritoneal fluid | Hepatic hydrothorax |
| Iatrogenic fluid instillation | Misdirected central venous catheters |
CLINICAL MANIFESTATIONS OF PLEURAL EFFUSIONS
A comprehensive medical history and physical exam of the chest are critical elements in guiding the diagnostic workup of a patient with a pleural effusion. Symptoms related to a pleural effusion depend on its size and the state of underlying lung function. Shortness of breath at rest and on exertion, as well as cough are the main symptoms. However, the clinical presentation can be dominated by other respiratory symptoms if there is coexisting asthma, COPD, or pneumonia. The primary physical findings in patients with a pleural effusion are dullness to percussion over the affected area, decreased tactile fremitus, diminished breath sounds, and occasionally egophony at the upper level of large effusions (Chap. 14). Very large pleural effusions can be associated with bulging of the intercostal spaces or contralateral shift of the mediastinum.
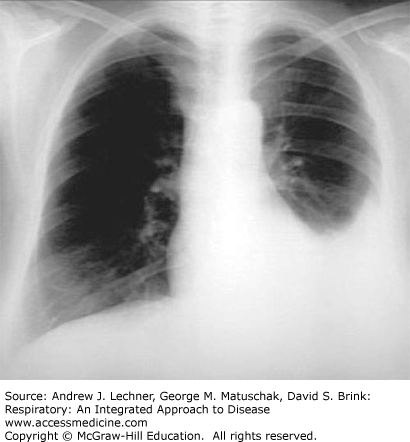
Radiographic evaluation is important in confirming the presence of a pleural effusion, as well as in formulating the most likely differential diagnostic possibilities. On upright chest radiographic frontal views, larger pleural effusions usually manifest as a meniscus-shaped density in the costophrenic sulcus (Fig. 29.3); smaller effusions may appear only as blunting of the costophrenic sulcus in upright views. Generally, at least 300-500 mL of fluid in the pleural space is required for visualization on x-ray. Accordingly, lateral views are useful for confirmation, as are supine portable AP films that can disclose free-flowing effusions as unilateral increases in radiographic density because of gravitational layering of the fluid over the involved hemithorax.
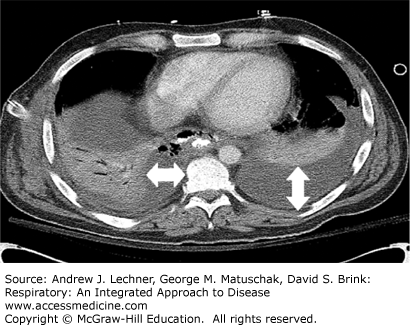
In such situations, pleural effusions are manifest as a diffuse, generalized haziness over the ipsilateral lung field with visible underlying lung vascular markings, together with absence of other signs of pulmonary consolidation such as air bronchograms (Chap. 15). The main value of lateral decubitus films is to confirm the presence of a pleural effusion as well as to evaluate the possibility of a loculated fluid collection. Fluid that is free-flowing will layer to a thickness of at least 10 mm on such decubitus views, in contrast to loculated fluid collections that have a similar appearance and shape regardless of the patient’s position. Chest CT imaging is the most sensitive method to detect pleural fluid collections and pleural disease (Chap. 15). Pleural effusions can easily be identified by their homogeneous lower density with variable degrees of lung compression (Fig. 29.4).