LEARNING OBJECTIVES
Learning Objectives
The student will be able to describe the three main inflammatory responses to pulmonary infection (intra-alveolar suppurative, interstitial mononuclear, and granulomatous) and identify characteristic microbiological etiologies for each.
The student will be able to distinguish bronchopneumonia from lobar pneumonia and identify specific etiologies of each.
The student will be able to describe the clinical settings in which a lung abscess can develop and identify microorganisms that can cause abscess formation.
The student will be able to distinguish primary tuberculosis from secondary (reactivation) tuberculosis, based on morphologic as well as clinical features.
The student will be able to recognize common fungi causing pulmonary infection.
The student will be able to describe Pneumocystis jiroveci pneumonia and recognize the causative microorganism.
As summarized in Chap. 10, the respiratory system has many defenses against infectious disease. The vibrissae in the nose and the mucociliary covering of the mucosa in the conducting portion of the respiratory system trap particles and micro-organisms, moving them cephalad to the pharynx to be expectorated or swallowed. Macrophages within the respiratory parenchyma phagocytize small particles and microorganisms and, if overwhelmed, recruit neutrophils from alveolar septal capillaries. Finally, immunoglobulin A in the mucosal secretions supports humoral immunity. In general, suboptimal humoral immunity and/or suboptimal nonimmune defense systems increase the risk for pyogenic bacterial infection, whereas suboptimal cell-mediated immunity increases the risk of infection by intracellular and low-virulence organisms, typically viruses and some bacteria. Other risk factors for the development of respiratory tract infection include decreased/absent cough reflex, reduced phagocytic/bactericidal activity of alveolar macrophages (eg, by alcohol, smoking, anoxia, oxygen intoxication), pulmonary congestion/edema, accumulation of airway secretions (eg, cystic fibrosis or distal to an obstruction), and mucociliary dysfunction, both congenital (eg, immotile cilia syndrome, Kartagener syndrome) and acquired (eg, viral illness, toxic effects of inhaled smoke).
This chapter will focus on the pathology of infectious disease and is organized by general morphologic similarities. There are three major morphologic patterns in infections of the respiratory tract: intra-alveolar accumulation of neutrophils, with or without abscess formation; interstitial expansion by mononuclear inflammatory cells; and granulomatous inflammation. Lung infections can also be organized based on their clinical settings rather than by their morphologic patterns (Table 34.1).
| Community-Acquired Acute Pneumonia | Community-Acquired Atypical Pneumonias | ||
| Streptococcus pneumoniae | Mycoplasma pneumoniae | ||
| Haemophilus influenzae | Chlamydia spp. | ||
| Moraxella catarrhalis | Coxiella burnetii (Q fever) | ||
| Staphylococcus aureus | Respiratory syncytial virus | ||
| Legionella pneumophila | Parainfluenza virus (children) | ||
| Klebsiella pneumoniae | Influenza A & B viruses (adults) | ||
| Pseudomonas spp. | Adenovirus, SARS virus | ||
| Hospital-Acquired Pneumonia | Aspiration Pneumonia (usually mixed flora) | ||
| Klebsiella spp. | Bacteroides spp. | Prevotella spp. | |
| Serratia marcescens | Fusobacterium spp. | S. pneumoniae | |
| Escherichia coli | Peptostreptococcus spp. | ||
| S. aureus | S. aureus | H. influenzae | |
| Pseudomonas spp. | Pseudomonas aeruginosa | ||
| Chronic Pneumonia | Necrotizing Pneumonia and Lung Abscess | ||
| Nocardia asteroides | Anaerobic bacteria | ||
| Actinomyces israelii | S. aureus | ||
| Mycobacterium tuberculosis | Klebsiella pneumoniae | ||
| Atypical Mycobacteria spp. | Streptococcus pyogenes | ||
| Histoplasma capsulatum | S. pneumoniae type 3 | ||
| Blastomyces dermatitidis | |||
| Pneumonia in the Immunocompromised Patient Setting | |||
| Cytomegalovirus | Pneumocystis jiroveci | ||
| Aspergillus spp. | Candida spp. | ||
| Mycobacterium avium-intracellulare | Usual bacteria, viruses, and fungi listed above | ||
CLINICAL CORRELATION 34.1
It is worth considering the term “pneumonia.” Some authors use the word when referring to pneumonitis (lung inflammation) due to a bacterial infection. Others use pneumonia to refer to any infectious pneumonitis, and others use it synonymously with “pneumonitis” without qualifiers. Whether a disease is more commonly referred to as pneumonia or as pneumonitis largely reflects traditional clinical usage rather than rational differentiation of the terms.
SUPPURATIVE BACTERIAL PNEUMONIA
In suppurative bacterial pneumonia, there is consolidation (ie, solidification) of lung parenchyma due to the accumulation of neutrophil-rich intra-alveolar exudate (Fig. 34.1). The consolidation of bacterial pneumonia is classically subclassified into two patterns: lobar pneumonia and bronchopneumonia (or lobular pneumonia).
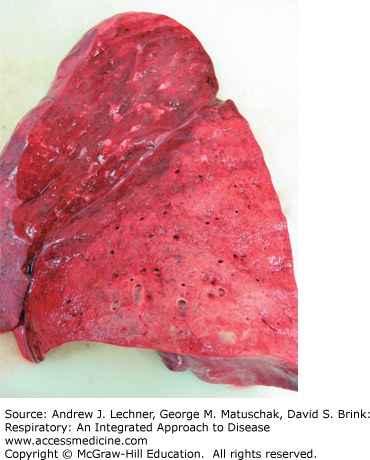
In lobar pneumonia, there is consolidation of contiguous airspaces, typically an entire lobe (Fig. 34.2). Lobar pneumonia is identifiable on an x-ray by a well-circumscribed radiopacity correlating to the affected lobe. Lobar pneumonia evolves through phases of congestion, red hepatization, gray hepatization, and resolution (Fig. 34.3).
FIGURE 34.2
Lobar pneumonia. Shown is the cut surface of a left lung with lobar pneumonia affecting the lower lobe. The lower lobe is markedly paler than the upper lobe. On palpation, the lower lobe would be consolidated with markedly reduced or absent crepitus. From Kemp et al. Pathology: the Big Picture, McGraw-Hill; 2008.
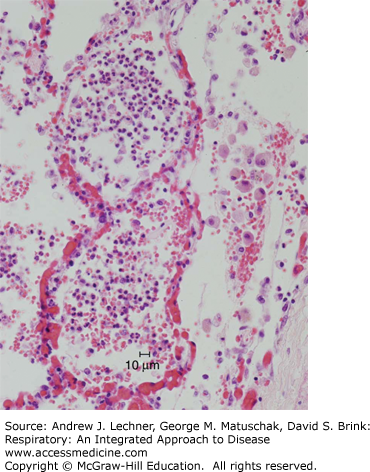
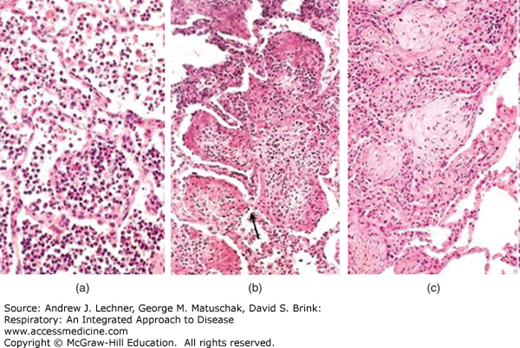
FIGURE 34.3
Phases of acute pneumonia. (a) Following the congestion phase (with few neutrophils), acute pneumonia enters the red hepatization stage, with alveoli filled with neutrophils and erythrocytes. (b) Subsequently, as the gray hepatization stage evolves, neutrophils and fibrin fill the alveolar spaces. (c) Following gray hepatization, the pneumonia can resolve or, alternatively, the intra-alveolar exudate can undergo organization, in which alveoli are filled by nodules of fibroblasts, collagen, and macrophages. From Kumar et al. Robbins and Cotran Pathologic Basis of Disease, 8th ed. Saunders-Elsevier, 2010.
During the congestion phase, the affected lobe is heavy, red, and boggy; histologically, there is vascular congestion, accumulation of intra-alveolar neutrophils, and pulmonary edema. Eventually, the lungs develop the consistency of liver (hepatization). Early in hepatization, the gross appearance is red, and the microscopic morphology comprises alveoli packed with neutrophils, erythrocytes, and fibrin; fibrous or fibrinopurulent pleural exudates are common in this phase. Later in the hepatization phase, the color of the lobe becomes gray as the fibrinous inflammatory exudate persists but becomes devoid of erythrocytes. As the inflammatory reaction resolves, the consolidated exudate is digested, leaving a granular semi-solid fluid to be resorbed. Due to the availability and efficacy of antibiotic therapy, lobar pneumonia is now rare.
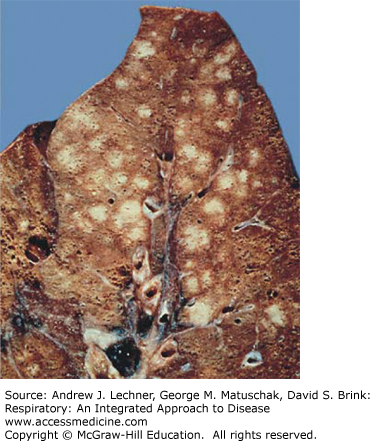
In bronchopneumonia, the pattern of consolidation is of noncontiguous airspaces and typically involves more than one lobe with a bronchiolocentric distribution. Grossly, the patches of consolidation are gray-red to yellow with a surrounding rim of hyperemia and edema (Fig. 34.4). Histologically, bronchopneumonia progresses through stages similar to lobar pneumonia (Fig. 34.3). Pleural involvement in bronchopneumonia is less common than in lobar pneumonia. Radiographically, bronchopneumonia is typified by multiple foci of radiopacity.
The clinical presentation of lobar pneumonia and of bronchopneumonia includes malaise, fever, and a productive cough, occasionally with pleurisy and pleural friction rub. Appropriate antibiotic therapy (Chap. 35) usually results in restoration of lung structure and function. Complications include abscess formation (see below) due to tissue destruction and necrosis, empyema (Chaps. 26 and 29), organization of the intra-alveolar exudate into solid fibrous tissue [Fig. 34.3(c)], and dissemination of the infectious organism, possibly leading to meningitis, arthritis, endocarditis, or sepsis. The mortality of patients hospitalized for bacterial pneumonia is less than 10%, with death typically related to the development of one of the aforementioned complications or due to the presence of a significant predisposition like debilitation or chronic alcoholism. There are numerous etiologies for suppurative bacterial pneumonia, many of which are briefly discussed below.
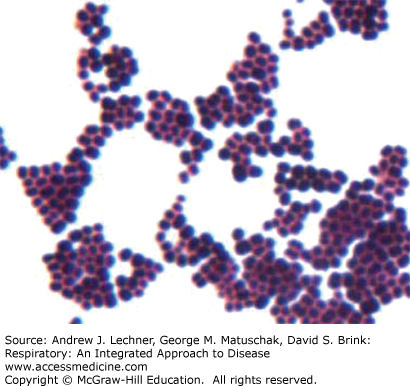
Streptococcus pneumoniae (previously Pneumococcus pneumoniae) is a gram-positive coccus; its cocci are typically paired. It is the most common cause of lobar pneumonia (>90% of cases) and is the most common cause of community-acquired acute pneumonia (15%-25% of cases). The most common microbiologic isolates are types 1, 2, 3, and 7. Of note, S. pneumoniae type 7 also causes lung abscess (see below). Staphylococcus aureus is another gram-positive coccus, but in contrast to the paired cocci of S. pneumoniae, the cocci of S. aureus are typically in “grapelike” clusters (Fig. 34.5). S. aureus typically causes bronchopneumonia with multiple abscesses (see below) but can cause lobar pneumonia. Risk factors for S. aureus pneumonia include recent measles infection in children, recent influenza infection in adults, and intravenous drug abuse.
Haemophilus influenzae is a gram-negative coccobacillus that generally causes bronchopneumonia that notably in children can be complicated by empyema (Chaps. 26 and 29) and extrapulmonary infection. Risk factors for H. influenzae pneumonia include recent viral infection, cystic fibrosis, chronic bronchitis, and bronchiectasis (Chaps. 20, 22, and 38). Moraxella catarrhalis is another gram-negative coccobacillus that generally causes bronchopneumonia. Risk factors for M. catarrhalis pneumonia include old age and chronic obstructive pulmonary disease (Chaps. 20 and 22).
Klebsiella pneumoniae is the most common gram-negative bacillus (rod) causing bacterial pneumonia. The morphology is that of bronchopneumonia or lobar pneumonia as well as lung abscess formation (see below). Clinically, K. pneumoniae pneumonia has an abrupt onset with a cough productive of gelatinous sputum. Risk factors for K. pneumoniae pneumonia include debilitation, malnourishment, and alcoholism. Recovery is often complicated by abscess formation, fibrosis, and/or bronchiectasis (Chap. 20), and K. pneumoniae pneumonia has a significant mortality rate, even with therapy. Pseudomonas aeruginosa
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree