Chronic Obstructive Pulmonary Disease |
Pathology of Chronic Obstructive Pulmonary Disease: Diagnostic Features and Differential Diagnosis
Chronic obstructive pulmonary disease (COPD) is a general name for the chronic airflow obstruction that develops most often as a result of chronic tobacco smoking, but also after exposure to biomass fuels. The pathology of COPD encompasses a variety of pathologic lesions in the airways, lung parenchyma, and pulmonary vasculature, and these lesions can be correlated, to a greater or lesser degree, with changes in pulmonary function tests and clinical appearances. In general, although the mechanisms involved are complex, airflow obstruction can be attributed largely to a marked increase in airway resistance secondary to a variable mix of structural abnormalities involving all or many of the compartments of the airway. However, in individual cases, it may be difficult to prove associations between physiologic abnormalities and pathologic changes. The Global Initiative on Obstructive Lung Disease (GOLD), recently revised,1 classifies patients with COPD purely upon indices of airflow and thus far there is only limited integration with pathologic findings.
This chapter presents the pathologic features of COPD and how these findings can be differentiated from other lesions associated with airflow obstruction.
HISTORY OF PATHOLOGIC DESCRIPTIONS OF COPD
The word emphysema is derived from Greek and means “to blow into,” hence “air-containing” or “inflated.” Although “voluminous lungs” and lungs “turgid particularly from air” were described respectively by Bonet in 16792 and Morgagni in 1769,3 the first description of enlarged airspaces in emphysema in the human, together with illustrations, was furnished by Ruysh in 1721,4 followed by Matthew Baillie in 1807, who not only clearly recognized and illustrated emphysema, but also pointed out its essentially destructive character.5,6
Laennec,7 writing in the early 1800s, made a number of seminal contributions to the basic descriptions of pathologic changes in COPD. He was the first to make a clear-cut distinction between interstitial emphysema and emphysema proper, and related the enlarged airspaces to the clinical syndrome of emphysema. He also recognized that air trapping and increased collateral ventilation were features of emphysematous lungs, and that the peripheral airways were the primary site of obstruction in emphysema. Furthermore, he noted that airspaces enlarged with increasing age, and he distinguished these changes from emphysema. He was the first to describe an association of emphysema with chronic bronchitis and to clearly describe the pathology of bronchiectasis.
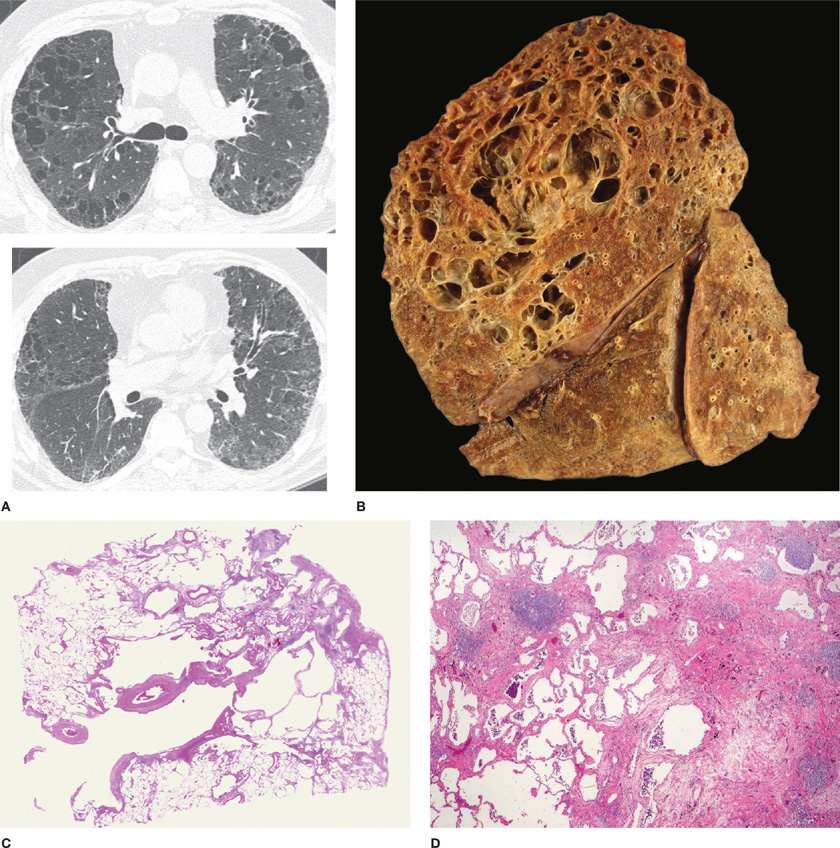
Little of major importance was added to the gross descriptive morphology of emphysema for almost the next 150 years. The foundation of modern knowledge of the pathologic anatomy of pulmonary emphysema was laid by J. Gough in 19528 when he described centrilobular emphysema and distinguished it from panlobular emphysema. The paper section technique developed by Gough and Wentworth9 was largely responsible for this advance, as it made examinations of sections of entire inflated lungs possible and simple (Fig. 39-1). A comprehensive microscopic description of emphysema was then provided by McLean,10,11 who demonstrated the relationship of destruction to inflammatory alterations of the bronchioles, and also discussed alterations of the vasculature.
Figure 39-1 Gough sagittal section. Paper mount. Normal lung.
LESIONS OF THE LUNG PARENCHYMA IN COPD: EMPHYSEMA
A major problem in describing the pathologic features of emphysema has been the lack of a generally accepted and easy to apply definition. In 1959, a Ciba Guest Symposium defined emphysema in anatomic terms as “a condition of the lung characterized by increase beyond the normal of airspaces, distal to the terminal bronchiole, either from dilatation or from destruction of their walls.”12 Subsequent definitions differed in that destruction of respiratory tissue became a requirement13–15: “Emphysema is a condition of the lung characterized by abnormal, permanent enlargement of the airspaces distal to the terminal bronchiole, accompanied by destruction of their walls.” This requirement separates emphysema from enlargement of airspaces unaccompanied by destruction, the latter now being termed overinflation.
Destruction has been similarly difficult to define in an unambiguous way. A committee of the National Institutes of Health16 proposed that destruction was present when “there was nonuniformity in the pattern of respiratory airspace enlargement so that the orderly appearance of the acinus and its components is disturbed and may be lost.” They recognized that emphysema was a subset of airspace enlargement defined as “an increase in airspace size as compared with the airspace of normal lungs. The term applies to all varieties of airspace enlargement distal to the terminal bronchioles, whether occurring with or without fibrosis or destruction.” While these definitions, when strictly applied, would eliminate airspace enlargement due to overinflation or failure of septation, they would not eliminate airspace enlargement due to reorganization of the airspaces, such as is found in honeycomb lung. This may be part of the confusion when combined emphysema and fibrosis is considered (see the section below).
CLASSIFICATION OF EMPHYSEMA
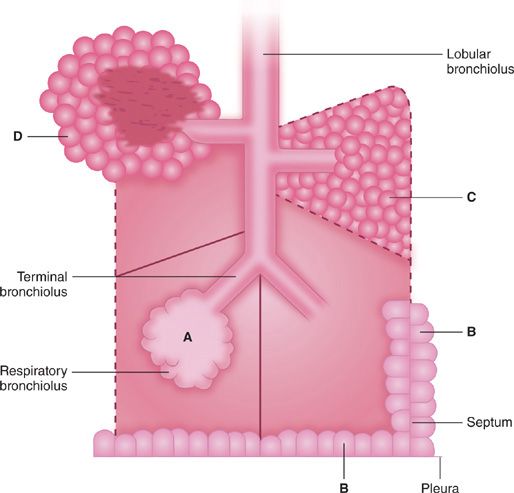
Not only is emphysema defined in terms of lung structure, it is also classified in similar terms; therefore, several anatomic definitions are important. The part of the lung involved in emphysema is the acinus, which is defined as the unit of lung structure distal to the terminal bronchiole (final generation membranous bronchiole) and that consists of three orders of respiratory bronchioles: a single order of alveolar ducts, followed by the alveolar sacs, and finally the alveoli. Alveolar ducts are entirely alveolated and characteristically contain smooth muscle around the mouths of their alveoli. While the walls of alveolar sacs are also formed entirely by alveoli, muscle is absent. Alveolar pores of Kohn (also known as vents, stomata, or fenestrae) are normal components of adult alveoli, responsible for collateral ventilation. However, they may also be an initial site of destruction in the development of emphysema, particularly centriacinar emphysema.
The acinus is a three-dimensional anatomic structure, but it cannot be easily identified by gross examination. What can be seen instead on the surface of lung slices is the secondary lobule of Miller, defined as the tissue bounded on four sides by interlobular septa or pleura (see Fig. 39-1). Lobules vary tremendously in size, but are generally 2 to 4 cm on a side, and contain between three to five acini. The terminal bronchiole and subtending respiratory bronchioles tend to be situated in the center of the lobule. For this reason “centrilobular” emphysema and “panlobular” emphysema are reasonable and widely used approximations for the more accurate “centriacinar” and “panacinar” emphysema (see below).
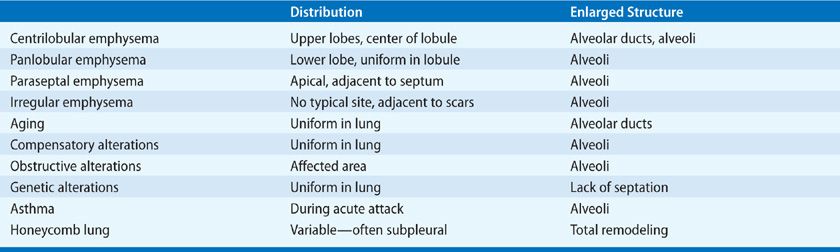
The ways in which the acini are involved determine the classification of emphysema. There are four recognized patterns (Fig. 39-2). The acinus (and lobule) may be more or less uniformly involved; this is panacinar (panlobular) emphysema. The proximal portion of the acinus (center of the lobule) may be dominantly involved; the best term for this lesion is proximal acinar emphysema, although the usual term is centrilobular or centriacinar emphysema. Alternately, the proximal portion of the acinus may be normal, and the distal part (alveolar sacs and ducts) may be dominantly involved. This is distal acinar emphysema, more commonly referred to as paraseptal emphysema since the lesion is accentuated along lobular septa where the peripheral parts of the acini lie. Finally, the acinus may be irregularly involved, producing irregular emphysema or paracicatricial emphysema, so called because it is usually associated with obvious adjacent scarring.
Figure 39-2 Anatomic varieties of emphysema. A. Centriacinar (centrilobular). B. Paraseptal (distal acinar). C. Panacinar (panlobular). D. Irregular (scar). The dashed lines mark the edge of the acinus. Only centriacinar and panacinar emphysema are commonly observed in COPD, although paraseptal emphysema often can be found in focal areas in lungs with centriacinar emphysema.
MORPHOLOGY OF EMPHYSEMA
 CENTRILOBULAR EMPHYSEMA
CENTRILOBULAR EMPHYSEMA
This destructive lesion of the respiratory bronchioles has a number of characteristic features on gross examination of the lung. In the classical lesion, the enlarged, destroyed respiratory bronchioles coalesce in series and in parallel to produce sharply demarcated emphysematous spaces, separated from the acinar periphery (the lobular septa), by intact alveolar ducts and sacs of normal size. The walls of the emphysematous spaces and adjacent tissue characteristically contain variable amounts of black pigment.
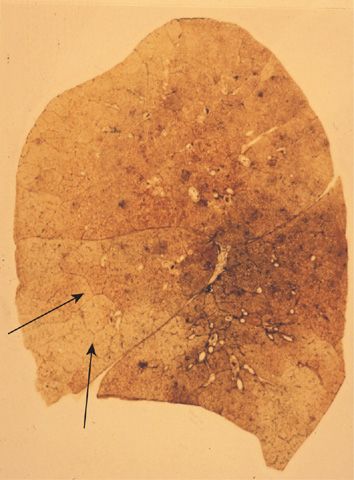
The lesions vary qualitatively as well as quantitatively even within the same lung. There is striking irregularity of involvement of lobules, and even within the same lobule.17,18 The lesions are usually more common and become more severe in the upper than in the lower zones of the lung (Figs. 39-3A and 39-4A,B).19–24 Most affected are the upper lobe, particularly the posterior and apical segments, and the superior segment of the lower lobe. In cases of severe CLE, the destruction proceeds toward the periphery of the lobule, and the distinction between CLE and PLE becomes blurred.
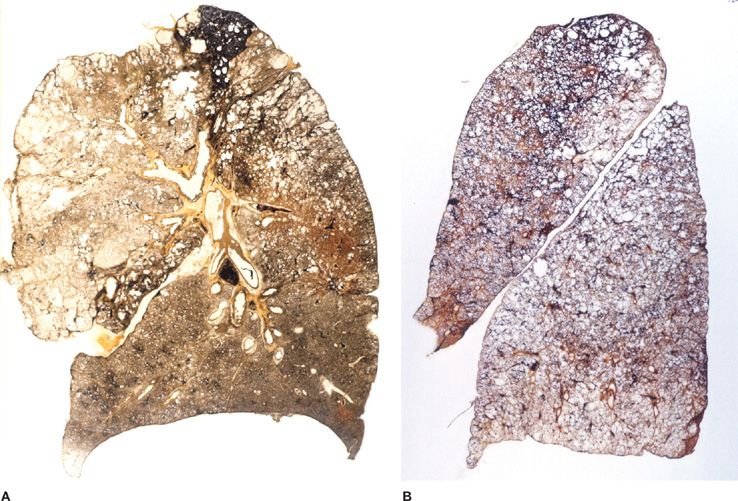
Figure 39-3 Pathologic subtypes of emphysema. A. Predominantly centriacinar emphysema. Emphysema is more severe in upper lobes. B. Predominant panacinar emphysema. Emphysema is more severe in the lower lobes.
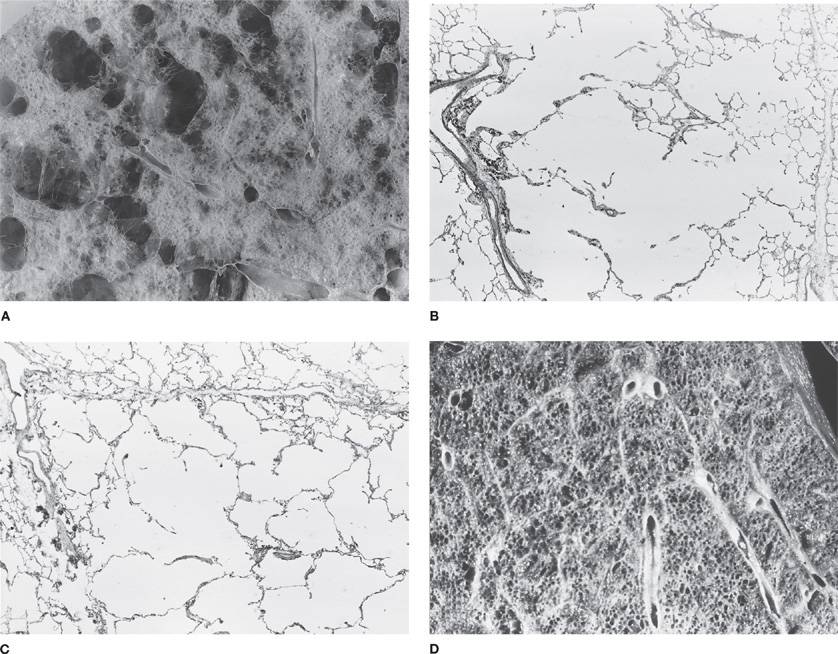
Figure 39-4 A, B. Gross and histologic sections illustrating centriacinar; and (C, D) panacinar emphysema. A. Cut surface from a lung with centriacinar emphysema showing holes in the center of lobules surrounded by relatively normal parenchyma. The severity varies among lobules. B. Microscopic section showing that the airspace enlargement in centriacinar emphysema is most marked adjacent to the abnormal respiratory bronchiole, corresponding to the center of the lobule. Also, some of the alveolar walls of the abnormal airspaces are thickened and fibrotic (H&E, ×16). C. Cut surface of a lung slice showing how the entire lobule is uniformly affected in panacinar emphysema. D. Microscopic section demonstrating that in panacinar emphysema, the airspaces adjacent to the lobular septa are enlarged to the same degree as those in the center of the lobule (H&E, ×16).
In CLE, alveolar pores are abnormal in size and shape, and occasionally contain epithelial debris and macrophages. Although there are numerous pores of variable size in the emphysematous areas,25 there are also increased numbers of pores in the grossly normal areas, and accentuation of these changes in the center of the lobule.26 Thus, it appears that in CLE the pores of Kohn are possibly the initial site of destruction.
There is increased cellularity in the alveolar walls of cigarette smokers,27 and when this has been quantified, the parenchyma in severe emphysema has increased numbers of neutrophils, macrophages, eosinophils, and both CD4 and CD8 T lymphocytes.28 There is also a significant inflammatory cell infiltrate in the airspaces in severe emphysema, with the same cell types increased.28 Although not readily apparent grossly or on standard histologic stains, use of histochemical stains or biochemical analysis demonstrates that collagen is increased in both centrilobular and panlobular emphysema.29–31
 PANLOBULAR EMPHYSEMA
PANLOBULAR EMPHYSEMA
The recognition of mild panlobular emphysema is very difficult. The normal lung has a very characteristic appearance when seen through a dissecting microscope: The multifaceted alveoli form a contrast to the larger, cylindrical conducting structures that are alveolar ducts and respiratory bronchioles. In panlobular emphysema the distinction between alveolar ducts and alveoli becomes lost as alveoli lose their sharp angles, enlarge, and then lose their contrast in size and shape with the ducts, resulting in simplification of the lung architecture, with formation of small box-like structures. As the process becomes worse, the architectural derangement becomes more obvious, with progressive effacement and loss of the orderly arrangement of the lung until little remains other than the supporting framework of vessels, septa, and bronchi. The best way to see panlobular emphysema grossly is to examine lung slices immersed in a water or fixative bath and then immediately after removal from the bath. The immersed specimen shows enlarged airspaces and, when the slices are lifted from the bath, panlobular emphysema can be suspected because the lung parenchyma “falls away” from the supporting structures and protrudes slightly above them. In contrast to centrilobular emphysema, panlobular emphysema is usually worse in the lower lobes (Fig. 39-3B).
Histologic examination is a sensitive method of recognizing panlobular emphysema. The pattern is again one of simplification with diminishing contrast between alveoli and alveolar ducts (Fig. 39-4C,D). Despite the greater extent of tissue destruction, in panlobular emphysema the pores of Kohn are more uniform and inconspicuous than those found in centrilobular emphysema.32
Panlobular emphysema is the characteristic lung lesion seen in α1-antitrypsin deficiency,33 but may also occur as a consequence of permanent obliteration of airways (obliterative bronchiolitis, constrictive bronchiolitis). Most often, obliteration of airways results in collapse of the distal lung parenchyma and dilatation of the bronchi proximal to the obliterated airways. This is the sequence of events in postinfective bronchiectasis. In some instances, however, the lung parenchyma does not collapse, but remains fully expanded or becomes emphysematous. The parenchymal sequel to bronchial and bronchiolar obliteration depends on the extent of the obliteration and the amount of collateral ventilation between adjacent airspaces distal to unobstructed airways. If collateral ventilation is present, then the units distal to the obliterated airways will remain expanded by virtue of the air reaching them by collateral ventilation, producing overexpansion and destruction of lung parenchyma beyond the obliterated airways. The terms Swyer–James or MacLeod syndrome are applied when this process affects most of one lung but spares the other.
 DISTAL ACINAR EMPHYSEMA: PARASEPTAL EMPHYSEMA
DISTAL ACINAR EMPHYSEMA: PARASEPTAL EMPHYSEMA
The original description of distal acinar emphysema is generally credited to Loeschcke,34 who described collections of subpleural bullae. It was Heard,24,35 however, who first noted that the lesions could extend into the substance of the lung, where they lay along the septa, and coined the term “paraseptal” emphysema. Since the distal part of the acinus (alveolar sacs and ducts) is dominantly involved, emphysema is most striking adjacent to the pleura (superficial emphysema or mantel emphysema), along lobular septa (paraseptal emphysema), at the margins of lobules and acini (periacinar emphysema), and along vessels and airways, which, when cut longitudinally, display a linear pattern. The characteristic morphology is that of multiple contiguous, enlarged airspaces, varying from <0.5 mm to >2 cm in diameter.
Paraseptal emphysema is usually limited in extent, and is found most commonly along the anterior and posterior parts of the upper lobe and along the posterior surface of the lower lobe. When extensive, it is usually more severe in the upper half of the lung. Gough has stressed that it is associated with fibrosis of the tissue between the enlarged airspaces, and this is certainly a common finding.36 Paraseptal emphysema is frequently found in association with centriacinar emphysema,20 but it is most known for its association with spontaneous pneumothoraces in young thin adults.37
 IRREGULAR EMPHYSEMA
IRREGULAR EMPHYSEMA
Irregular emphysema is logically named, because the acinus is indeed irregularly involved in it. Irregular emphysema is almost invariably adjacent to a scar, giving name to the synonyms scar or paracicatricial emphysema. Most scars within the lung are usually small and the emphysema is limited in extent. The severity of irregular emphysema depends on the extent of damage to lung tissue, and multiple scars through the lung may lead to multiple foci of irregular emphysema.
DIFFERENTIAL DIAGNOSIS OF EMPHYSEMA
 GAS TRAPPING
GAS TRAPPING
The lungs of an asthmatic who has succumbed during an attack are usually characterized by gas trapping, and thus remain inflated, with focal areas of atelectasis (Table 39-1). In a patient with longstanding asthma who has died from other causes, or has had a lung resection, there may still be areas of atelectasis. Focal bronchiectasis can be found also, particularly in the anterior segment of the upper lobe. However, parenchymal destruction is not a feature of asthma, and thus gross, microscopic, and morphometric analyses will all be normal in the chronic asthmatic.
 NONEMPHYSEMATOUS AIRSPACE ENLARGEMENT
NONEMPHYSEMATOUS AIRSPACE ENLARGEMENT
Although not part of the differential diagnosis of COPD, nonemphysematous airspace enlargement also occurs in infancy. In congenital lobar hyperinflation (emphysema), the lobes are overinflated rather than emphysematous, but in some instances they may be polyalveolar.38,39 Some other genetic abnormalities will also give enlarged airspaces, but this is due to failure of septation with a simplified rather than a destroyed alveolar framework.
At the other side of the age spectrum, the term senile emphysema was once used to describe the enlarged airspaces found in the aged. On gross examination, lungs round out with increasing age. An analysis of Gough sections showed increases in anteroposterior distance, height, perimeter, and area of the lung up to the age of 59 years. After this age, only the anteroposterior diameter continued to increase significantly, thus “rounding” the lung dimensions.40 This change is due to an increase in the volume proportion of alveolar duct air,41 with shallower and flatter alveoli,42 a process termed ductectasia. There is no evidence of lung destruction; thus, the condition does not fulfill the criteria for emphysema.
If a part of the lung collapses or is removed, the remaining lung can expand to fill the increased amount of space available, a process known as compensatory overinflation. The exact way that this happens and the limits of the process are unknown. However, no tissue destruction has occurred and, by definition, this is not emphysema. It is not clear how much larger the overinflated lung can become, or how it expands to reach the new and larger volume. It is generally thought that the possible extent of overinflation is modest and that all the parts of the acinus are equally expanded.
Obstructive overinflation can occur in adults, and two mechanisms may be involved. In one, the obstruction in the bronchus may act as a ball valve, so that air enters on inspiration but does not leave on expiration. Alternatively, the bronchus may be completely obstructed and air may be trapped behind channels of collateral ventilation. Whatever the mechanism, the affected part of the lung can expand considerably. Obstructive overinflation differs in a number of ways from compensatory overinflation, although, in both, the lung contains too much air per unit of lung and lung tissue.
 HONEYCOMB LUNG
HONEYCOMB LUNG
The airspace enlargement that occurs in cryptogenic fibrosing alveolitis (usual interstitial pneumonia [UIP]) and other fibrotic lung diseases could possibly be confused with emphysema. While honeycomb spaces are enlarged airspaces, they are the result of parenchymal remodeling with formation of new airspaces, rather than destruction of normal airspaces, and thus have thickened and irregular walls with none of the structure of an acinus. They are lined by bronchiolar epithelium, and often contain mucus; the walls have abundant and well-collagenized connective tissue, which may also contain impressive amounts of muscle and sometimes fat. There is usually interstitial inflammation in the form of varying degrees of lymphocytic and plasma cell infiltration.
 COMBINED EMPHYSEMA AND FIBROSIS
COMBINED EMPHYSEMA AND FIBROSIS
Despite the definition of emphysema, which limits fibrosis, the significance of a mixture of fibrosis and emphysema has recently been reevaluated in relationship to its clinical, radiologic, and pathologic components.43 The problem is that people who smoke cigarettes not only can develop respiratory bronchiolitis-interstitial lung disease (RB-ILD), but also have a higher incidence of developing UIP (idiopathic interstitial fibrosis), and mixtures of these with emphysema are not uncommon. When the combination of emphysema and UIP occurs, lung volumes can be preserved, but the diffusing capacity becomes markedly decreased, and pulmonary hypertension develops, with its associated significant negative prognosis. CT scans generally show centrilobular or mixed centrilobular and paraseptal emphysema in the upper lobes, with increased reticular markings and honeycomb remodeling in the lower lobes. Pathologically, there is both gross and microscopic emphysema and interstitial fibrosis with fibroblast foci in the areas of active fibrosis.44 We have recently reviewed this topic with a focus on the pathologic differential diagnosis (Fig. 39-5A–D).45
Figure 39-5 Combined fibrosis and emphysema in a case of chronic (fibrotic) hypersensitivity pneumonitis. A. Computed tomography scan from upper zone (top) shows emphysema and a suggestive of reticulation; lower image from midlung zone shows extensive reticulation indicating the presence of underlying fibrosis. B. Gross photo (sagittal slice) from this case showing marked upper zone emphysema, with fibrosis evident in the most posterior portion of the upper lobe, and the posterior portions of the lower lobe. C. Whole mount from the upper lobe. There are large emphysematous spaces, several with extensive surrounding fibrosis; at higher magnification, many of these fibrotic rims had fibroblast foci (not shown), indicating that this process is really interstitial fibrosis stretched around pre-existing emphysema. D. Image from lower lobe showing a UIP-like area, a common finding in chronic hypersensitivity pneumonitis. Elsewhere there were noncaseating granulomas (not shown). (Reproduced with permission from Wright JL, Tazelaar H, Churg A. Fibrosis with Emphysema. Histopathology. 2011;58(4):517–524 (This document was published in 1993. Certain aspects of this document may be out of date and caution should be used when applying the information in clinical practice and other usages).)
LESIONS OF THE LARGE AIRWAYS IN COPD
The majority of studies in this area have focused upon the lesions present when the clinical signs and symptoms of chronic bronchitis are also present.
 GROSS FINDINGS
GROSS FINDINGS
Gross lesions in the large airways are few and subtle. Bronchial pits are the dilated openings of one or more mucus glands into the epithelium. They are most often found along the margins of the cartilaginous rings and at the bifurcations of the airways. In nonbronchitis the pits can be seen using a hand lens or a dissecting microscope, but in chronic bronchitis, the ducts may be distended with mucus and the mucus may protrude into the lumen of the bronchus and be visible grossly. It is not correct to refer to these as diverticula. First, these are protrusions of normal ducts; and second, they do not extend through all of the muscle coats of the bronchial wall.
While enlarged bronchial pits are the most obvious gross lesions in COPD, careful examination of lung specimens will show that the bronchi do not taper progressively as they approach the pleura,46 and they also display prominent circular ridges, probably due to bands of hypertrophic smooth muscle.47,48 Gross mucus may be present in the airway lumen, particularly in subjects with chronic bronchitis.49
 MICROSCOPIC FINDINGS
MICROSCOPIC FINDINGS
The intraluminal mucus found in the airways of subjects with COPD contains a mixed population of epithelial cells and acute and chronic inflammatory cells; large numbers of neutrophils can be found during an exacerbation.
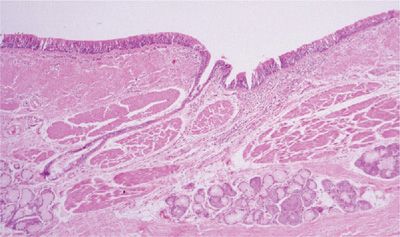
Detailed microscopic analysis of the large airways in COPD reveals alterations in the entire airway wall (Fig. 39-6). Epithelial changes are mild in degree and are not necessarily consistent from patient to patient. Epithelial sloughing can occur, but in most instances the epithelium is generally intact and shows only mild goblet cell or squamous cell metaplasia, both of which appear to be more marked if the subject has symptoms of chronic bronchitis.50,51 The reticular basement membrane thickness is within the normal range.
Figure 39-6 Large airway from a subject with chronic bronchitis. The overall wall is thickened with inflammation and fibrosis, and there is prominence of the smooth muscle in addition to the bronchial mucus glands.
The thickness or area of mucus glands in subjects with COPD in general, or chronic bronchitis in particular, is increased over a population mean, but has a distribution that extensively overlaps that of normals and asthmatics.52–55 Interestingly, there appears to be a decreased percentage of serous acini in these glands, a feature that apparently does not occur in asthma (discussed below).56
Thickening of the inner wall (area internal to the muscular layer) appears to be the most consistent component of airway wall thickening in the large airways of subjects with COPD, and appears to be generalized.57,58 This increase in thickness can be partially attributed to edema and hyperemia of the bronchi,59 but is also due to an increase in fibrous tissue or other matrix proteins.
In the large airways of subjects with COPD, increases in the thickness of the muscular layer have not been consistently identified. Although some studies60 have found that the average proportion of muscle in main, lobar, and segmental bronchi was approximately doubled in patients with chronic bronchitis and airflow obstruction, others have found that a substantial number of patients fell within the normal range.54,55,61
Alteration in the amount of cartilage in COPD does not appear to be a consistent finding. While some studies59,62,63 described cartilage atrophy in chronic bronchitis and/or emphysema, or circumferentially arranged cartilage that extended farther distally in nonbronchitis than bronchitis,64 this was not supported by other reports.54,65 However, histologic signs of cartilage damage, as judged by loss of cellular or pericellular metachromasia and vacuolated or empty lacunae can be consistently identified.66
The large airways in COPD show a mild, usually mixed, inflammatory infiltrate. Bronchus-associated lymphoid tissues (BALT) is not consistently found, but its frequency appears to be considerably higher (82%) in smokers than nonsmokers (14%).67 Bronchial biopsy analysis consistently shows an increase in CD8 T cells, with eosinophils and neutrophils found during exacerbations (reviewed in Refs.50,68). Chronic inflammation can also be found around the bronchial glands, particularly in subjects with chronic bronchitis.69
DIFFERENTIAL DIAGNOSIS
 ASTHMA
ASTHMA
In asthma the large airways are not dilated, but mucus plugs are classically identified in the large airways of subjects with fatal or near-fatal asthma,70 and the mucus may be continuous with that present in the ducts of the mucus glands (Table 39-2). Visible bronchial pits are not a standard feature of asthma, and although the airway wall may be thickened, this is usually not apparent grossly.