Pathology of Carcinoma of the Lung
Thomas W. Shields
Derivation of Tumor Cell Types
In the normal lung, stem cell activity, according to Otto and Wright,204 involves three cell types: (a) the basal cell of the bronchi and trachea, (b) the Clara cell of the bronchioles, and (c) the type II pneumocyte of the alveoli. These authors further note that stem cell activity of secretory cells remains unproved and that this is likewise true of the neuroendocrine cells. In tumor histogenesis, the role of one or more of these cells remains unanswered. Many investigators have implicated one or more of these cell types, but no unified concept has evolved. At present, there are two main hypotheses of lung cancer pathogenesis. The first is that of a pluripotent stem cell from which the various tumor phenotypes arise by differentiation. The second is the multiple primary hypothesis in which the various phenotypes arise from different cells of origin. There has been no universal acceptance of either hypothesis. Brambilla,29 in reviewing the basaloid carcinomas of the lung, has noted that “basaloid carcinoma cells show the morphological, immunophenotypical, and ultrastructural features of totipotent reserve or basal cells, which have the propensity for further multi-directional differentiation among squamous, glandular, or even neuroendocrine pathways.” This observation, if confirmed by others, would help to explain the heterogenicity of tumor cell types within a given tumor. Actually, heterogenicity is the rule rather than the exception in most lung tumors. Yesner303,305 reported the frequent finding of areas of non-small-cell tumor phenotypes in small-cell lung cancers (SCLCs), as well as the presence of neuroendocrine differentiation in non-small-cell lung cancers (NSCLCs). Roggli and colleagues239 observed that only 34% of tumors studied in their investigation were homogenous for one cell type of cancer and that 45% of tumors contained two major histologic cell types. Of the SCLCs reviewed, at least half contained an area of another major histologic type. What triggers the basal cell (indifferent/indeterminate basal or suprabasal intermediate cell, the small mucous granule cell) in the tracheobronchial epithelium to differentiate into one or more of the multiple tumor phenotypes is unknown. Minna,164 however, has suggested that malignant transformation under various stimuli may result in different phenotypes, depending on the type of mutation acquired on key genes that control proliferation, differentiation, and apoptosis.
Pathology
Gross Characteristics
Bronchial carcinoma occurs more frequently in the right lung than in the left in a ratio of approximately 6 to 4. The upper lobes are involved more often than the lower lobes, and the middle lobe is involved the least frequently. In the upper lobes, the tumor is most likely located in the anterior segment, although the other segments are not spared as the site of origin.
The anatomic site of origin of the tumor may be classified as (a) the central zone, including the main stem, lobar bronchi, and primary segmental bronchi of the lower lobe; (b) the segmental or intermediate zone, including the third-, fourth-, and possibly the fifth-order segmental bronchi; and (c) the peripheral zone, which includes the remainder of the distal bronchi, bronchioles, and alveoli. In the radiographic localization of lung tumors, zones 1 and 2 may be considered the central area and zone 3 the peripheral area.
According to Meyer and Liebow,161 approximately 50% to 60% of carcinomas of the lung originate in the peripheral area. Of those carcinomas that arise in the central and segmental zones (the central area), 20% to 40% arise in the former and 60% to 80% in the latter.
Grossly, the tumors in the central and segmental zones appear as firm, irregular masses of varying size. Intraluminal growth may occlude the bronchial lumen partially or completely, although obstruction may also be caused by circumferential narrowing of the lumen. Extrabronchial spread may extend for a variable distance into the adjacent lung parenchyma. The tumor is generally homogeneous, with a whitish gray cut surface. The endobronchial surface is typically ulcerated. Atelectasis or secondary inflammatory change—including secondary bronchiectasis, pneumonitis, or lung abscess distal to the site of the tumor—is frequently present.
The peripherally located tumors are firm and irregular and may or may not appear to be demarcated from the surrounding lung tissue. The cut surface is homogeneous. The smaller lesions are usually solid, although the larger ones may reveal central necrosis with cavitation. Umbilication or puckering of the overlying adjacent visceral pleura is often present. The blood supply of both the centrally and peripherally located bronchial carcinomas is from the bronchial arteries.
Table 104-1 Common Carcinomas of the Lung | ||
|---|---|---|
|
Histologic Classification
Many histologic classifications of lung tumors have been suggested. A modified classification from the original suggested by Moori173 is shown in Table 104-1. This classification is less cumbersome than that proposed by the World Health Organization and International Association for the Study of Lung Cancer published in 1999 by Travis and associates.271 In clinical practice it is common to separate these tumor types into either small-cell cancer (SCLC) or non-small-cell cancer (NSCLC). Furthermore, the SCLCs belong to the spectrum of neuroendocrine tumors of the lung. Azzopardi14 and Bensch and associates20 were among the early investigators who established the recognition of the neuroendocrine features of these tumors. Subsequently, the initial classification has undergone numerous modifications, with better delineation and characterization of the various subtypes in the neuroendocrine tumor classification (Table 104-2). Arrigoni and colleagues8 identified the atypical carcinoid tumors. Gould and coworkers,79,80 incorporating the increased knowledge concerning these tumors, suggested that the atypical carcinoid tumors be termed well-differentiated neuroendocrine carcinomas. They further suggested the term neuroendocrine carcinoma of the intermediate cell type be applied to all types of poorly differentiated neuroendocrine tumors that were distinct from either the atypical carcinoids or the small-cell type of neuroendocrine tumors. Travis and associates272 proposed a new category of these tumors: the large-cell neuroendocrine tumors. The latter authors and colleagues273,274 have further defined the criteria for the classification of the large-cell neuroendocrine tumors. Wick293 suggests that a new terminology would be better for the neuroendocrine lung tumors: (a) grade I, well-differentiated neuroendocrine tumor (carcinoid); (b) grade II, moderately differentiated neuroendocrine tumor (atypical carcinoid); and (c) grade III, poorly differentiated neuroendocrine tumor (i) of the large-cell type and (ii) of the small-cell type.
Preinvasive Lesions
Squamous dysplasia/carcinoma in situ, atypical adenomatous hyperplasia, and diffuse idiopathic pulmonary neuroendocrine cell hyperplasia are the three preinvasive lesions included in this category. The first two are related to squamous cell carcinoma and to bronchioloalveolar/adenocarcinoma, respectively. The third, neuroendocrine cell hyperplasia, is believed to be the precursor lesion for carcinoids and is discussed in Chapter 124. Despite earlier speculation that carcinoid tumorlets might represent early or in situ SCLC, Flieder and Vazquez71 have noted that there is no established relationship or association between these two lesions.
Squamous Cell Dysplasia/Carcinoma in Situ
Grossly, in situ carcinoma appears as an area characterized by a loss of the normal longitudinal folds of the mucosa of a major or even a segmental bronchus. The mucosal area involved may be thickened and erythematous. Microscopically, the in situ carcinoma reveals full-thickness cytologic atypia with an increased nuclear/cytoplasmic ratio. The nuclei are hyperchromatic, and mitoses may be present. These changes do not extend beyond the basement membrane. Often such changes are associated with frank squamous cell carcinoma, but—as observed by Auer and associates13—complete regression of an isolated in situ lesion may occur.
Atypical Adenomatous Hyperplasia
Atypical adenomatous hyperplasia (AAH) is a proliferation of bronchioloalveolar cells that is thought to be a precursor to adenocarcinoma. According to Ritter,238 AAH resembles the nonmucinous variant of bronchioloalveolar carcinoma (BAC). Initially, these lesions were identified pathologically in lungs resected for adenocarcinoma. Rao and Fraire223 noted that these
areas of hyperplasia are present in up to 20% of resected lung cancer specimens as an ill-defined peripheral nodule, most often multiple in number. The size of the lesions, according to Weng and colleagues,291 was noted to range from 1 to 10 mm, with the majority being ≤5 mm in size. With the advent of helical computed tomography (HCT) for lung cancer screening, many more small lesions <10 mm in size are being identified. Such lesions may be further analyzed with the use of high-resolution CT (HRCT), as reported by Reeves and Kostis.225,226 The resulting images contain a great deal more information, and the size and shape of the lesion can be precisely measured and recorded. The opacity of the AAH lesion is indeterminate but at times may be “ground glass” in appearance.
areas of hyperplasia are present in up to 20% of resected lung cancer specimens as an ill-defined peripheral nodule, most often multiple in number. The size of the lesions, according to Weng and colleagues,291 was noted to range from 1 to 10 mm, with the majority being ≤5 mm in size. With the advent of helical computed tomography (HCT) for lung cancer screening, many more small lesions <10 mm in size are being identified. Such lesions may be further analyzed with the use of high-resolution CT (HRCT), as reported by Reeves and Kostis.225,226 The resulting images contain a great deal more information, and the size and shape of the lesion can be precisely measured and recorded. The opacity of the AAH lesion is indeterminate but at times may be “ground glass” in appearance.
Table 104-2 Comparison of Classifications of Neuroendocrine Tumors of the Lung | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
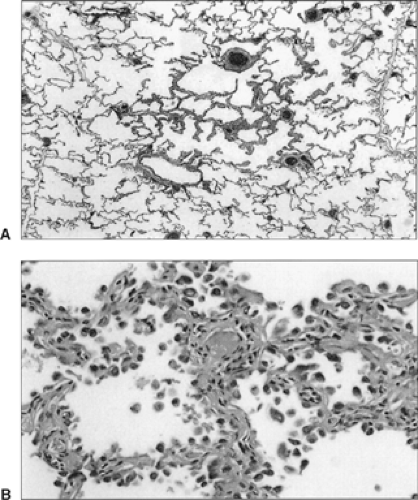
Histologically, the lesion appears as a zone of alveolar wall thickening as the result of the alveoli being lined by a variable number of cuboidal or columnar cells (Fig. 104-1A,B). Kerr121 notes that many of the cells have a hobnail or pear shape, reminiscent of Clara cells, while others show nuclear inclusions as seen in type II pneumocytes. There is variable nuclear pleomorphism, but it is most often mild in nature. There is a lack of any central fibrosis, and mucin production is absent. Kitamura and associates125 have classified these atypical adenomatous hyperplasias as low-grade, high-grade, and carcinoma-like. The proliferative potential and p53 expression increase as the grade advances. Likewise, Kitamura and colleagues126 recorded that the nuclear area and lesion size increased as the dysplastic lesion progressed to a bronchioloalveolar carcinoma; also, an increase in the level of carcinoembryonic antigen (CEA) was seen as these changes occurred. Any lesion >5 mm should be considered as a probable carcinomatous tumor. Vazquez and Flieder279 have stressed that the distinction between atypical adenomatous hyperplasia and bronchioloalveolar carcinoma is extremely difficult and subjective. The interpretation between a benign lesion and a carcinoma by examination of a fine-needle aspiration of such lesions is not possible in the absence of finding a diagnostic malignant cell. If no malignant cell or cells are identified, a tentative diagnosis of atypical adenomatous hyperplasia may be made. However, the lesion should be reevaluated by HRCT in 4 to 6 months to determine whether it is stable or if growth has occurred. If growth is apparent, the degree and rate of growth can be established by the techniques described by Yankelevitz and associates.297,298 Growth implies the presence of cancer, therefore further diagnostic evaluation or removal of the lesion is indicated.
As a consequence of an increased use of routine helical examination of the lungs and evaluation of any lesion identified by HRCT, a subset of small lesions has been identified as being solitary pure ground-glass opacities. Any areas of fibrosis or scarring exclude the lesion from this category of indeterminate masses. The natural history of these pure ground-glass opacities has heretofore been unknown. In an attempt to evaluate the natural history and characteristics of these opacities, Kodama and associates127 investigated and reported 49 patients with a solitary pure ground-glass opacity in the lung discovered by HRCT. Of these individuals, 39 had undergone surgical resection within 19 months of the initial identification of the lesion; 34 had BAC, 4 had adenocarcinoma, and 1 had AAH. The average diameter of all lesions was 13.3 mm, but in the 4 patients with adenocarcinoma, the average diameter was 27 mm. Nineteen additional patients with pure ground-glass opacities (17 patients with solitary lesions, 1 patient with two lesions, and 1 with many lesions) were observed for a period of 2 years or longer. Reasons for observation varied, but eventually 10 of the patients agreed to have their lesion removed; nine others elected continued observation. Overall, in the 19 patients the lesion increased >5 mm in size in 5 patients, increased slightly in 6, and showed no change in 8. It is of interest that six of the patients who eventually elected to have a resection had a history of a previously resected cancer (five in the lung and one in the kidney). Of the 10 lesions resected, the largest (25 mm) was an adenocarcinoma, 4 lesions were BAC, 1 was AAH, 3 showed the presence of a lymphoproliferative disorder, and 1 revealed the presence of pulmonary fibrosis. Of note is that four of the five patients with proven cancers had a prior history of a previously resected lung cancer. From these data, although not statistically significant, it seems apparent that any pure ground-glass opacity >10 mm in size that shows evidence of growth over a period of observation or is identified in a patient with a history of lung cancer should be strongly considered for resection despite the observation that a prolonged period of observation may not be excessively detrimental. Watanabe and colleagues287 carried out limited resection in 17 patients with pure ground-glass attenuation on HRCT. All were pure BAC
lesions and no recurrence or death occurred on short-term follow-up (median follow-up time was 32 months).
lesions and no recurrence or death occurred on short-term follow-up (median follow-up time was 32 months).
Non-Small-Cell Tumors
Squamous Cell Carcinoma
Gross Features
The squamous cell tumors constitute approximately 20% to 35% of all lung carcinomas. Until the latter half of the twentieth century, squamous cell carcinoma was the most common cell type of lung cancer throughout the world; but in the United States and Japan, this cell type is now less common than adenocarcinomas of the lung. However, squamous cell tumors remain the most common lung cancer in Europe and most other countries of the world. The cause of the changing incidence in the United States and Japan is obscure but most likely involves the change in smoking habits and the demographics of smoking in these two countries.
Squamous cell carcinomas may occur in either the central or peripheral areas of the lung, although more than two-thirds are found in the central area. Squamous cell tumors grow relatively slowly and tend to metastasize late. The centrally located lesions tend to extend both intrabronchially as well as peribronchially; therefore the lumen is frequently constricted by extrinsic pressure but has a grossly normal-appearing mucosal pattern. The obstructing tumors are often associated with obstructive pneumonitis, distal pulmonary collapse, and consolidation. As they enlarge, the peripherally located squamous cell tumors tend to undergo central necrosis with resultant cavitation. This may occur in 10% to 20% of the peripheral lesions.
Small, superficial squamous cell tumors of the bronchial mucosa, although not a separate variety, deserve mention. These tumors are radiographically occult and may occur in both the larger mainstem and lobar bronchi as well as in the more distal divisions of the bronchial tree. The more proximal lesions may be identified by bronchoscopic examination as abnormal areas in the otherwise normal-appearing bronchial mucosa. Some of these tumors are only carcinoma in situ, but many are invasive lesions, most of which do not extend beyond the bronchial wall. The length, gross area of involvement, and depth of extension determine whether these are aggressive lesions (see “Lymphatic Metastasis,” later in this chapter).
Microscopic Features
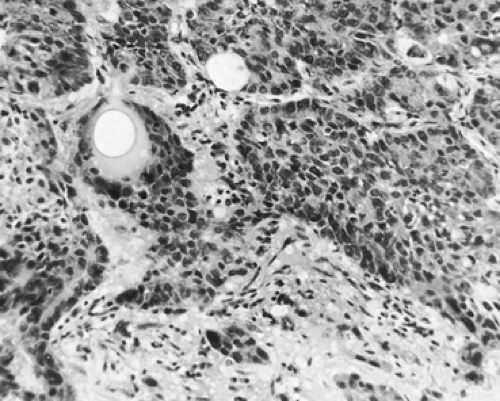
The well-differentiated squamous cell tumors have polygonal or prickle-type cells, stratification, and intercellular bridge formation (Fig. 104-2). Individual cells keratinize or tend to form epithelial pearls or both; the nuclei may be uniform, pleomorphic, or giant. The moderately differentiated tumors have polygonal or prickle-type cells, stratification, intercellular bridge formation, and some keratinization. Rarely, squamous cells can take on a spindle shape, which makes the tumor look sarcomatous histologically. The poorly differentiated tumors are composed predominantly of anaplastic cells, with little but still distinct evidence of intercellular bridge formation, individual cell keratinization, or both. A tumor without these latter features should not be placed in this category.
Electron Microscopic Features
Tumor cells frequently have degenerated and bizarre mitochondria and more free ribosomes, fewer profiles of granular endoplasmic reticulum, and more lipid than normal cells. The Golgi apparatus is usually poorly developed. Squamous cell carcinomas are composed mainly of polygonal cells with distinct cell membranes and numerous desmosomes between adjacent cells. Tonofilaments, keratohyalin granules, and some keratin pearls are dominant features. No neuroendocrine granules, as a rule, or mucin are seen.
Immunohistochemical Features
Squamous cell carcinomas are readily stained by polyclonal antibodies to epidermal-type cytokeratin, as noted by Blobel24 and Gould78 and their associates. Schaafsma and Ramaekers249 identified that keratins 4, 8, and 13 through 18 of Moll and colleagues’170 catalog are present. Many of these keratins are found in other lung tumors; keratin 14 is found in all squamous cell tumors but not in pulmonary adenocarcinomas or in any of the neuroendocrine tumors. Leu-7 antigen is also strongly associated with a squamous phenotype. Also, these tumor cells are stained with antibodies to epithelial membrane antigen, CEA, and desmosomal plaque proteins, but these are not unique to squamous cell differentiations. Other molecular markers have been identified in varying percentages and varying quantities in squamous cell tumors by numerous investigators including D’Amico and associates49,50,51,52,53,54,55,56,57,58,59,60 at Duke University. These include markers related to cell growth stimulation (EGF and Erb B2), cell cycle regulation (Rb and Ki-67), interference with apoptosis (p53 and bcl-2), tumor angiogenesis (factor VIII), invasive cell adhesion (STN and CD-44), and adhesions factors (Eph-A2 and E-cadherin). Other molecular and biological markers, as well as those listed, are discussed in more detail in Chapter 106. Last, Berendsen and colleagues21 observed that neuroendocrine differentiation status could be identified by monoclonal antibody–based immunohistologic procedures in a small percentage of squamous cell carcinoma cells.
Exophytic Endobronchial Squamous Cell Carcinoma
Sherwin251 and Dulmet-Brender62 and their coworkers described exophytic endobronchial squamous carcinoma as an uncommon type of squamous lung cancer with a papillomatous, polypoid, or verrucous growth pattern. This tumor has a gray-white granular to papillary appearance and tends to fill and obstruct the bronchus. Microscopically, these tumors have a verrucous, papillary, or polypoid growth pattern with an underlying fibrovascular stalk. The epithelial cells are malignant squamous cells with intercellular junctions (prickle cells) and possible keratinization. A lymphoplasmacytic reaction may be present in the underlying connective tissue. The squamous cells usually show superficial focal invasion into the bronchial wall, but some may be only in situ carcinoma. The electron microscopic and immunohistochemical findings in these tumors are similar to those of the typical squamous cell carcinomas.
Adenocarcinoma
Gross Features
Adenocarcinomas account for approximately 30% to 50% of all carcinomas of the lung. Vincent and associates282 in 1977 reported an increasing incidence of adenocarcinoma; they identified more adenocarcinomas than squamous cell tumors in 1,682 lung tumors studied. This observation was confirmed by numerous investigators, including Yesner and Carter,306 who reported that in an all male population of veterans in a two-decade period, a 7% increase in adenocarcinomas of the lung and an associated decrease of 4% in squamous cell tumors and a 3% decrease in small cell carcinomas occurred. Amemiya and Oho5 noted a similar preponderance of adenocarcinoma in the Japanese. Travis and colleagues275 reported that the incidence of adenocarcinoma is 40%, that of squamous cell carcinoma 30%, that of large cell carcinoma 10%, and that of small-cell lung cancer 20%, thus establishing adenocarcinoma as the most common histologic cell type of lung cancer at this time.
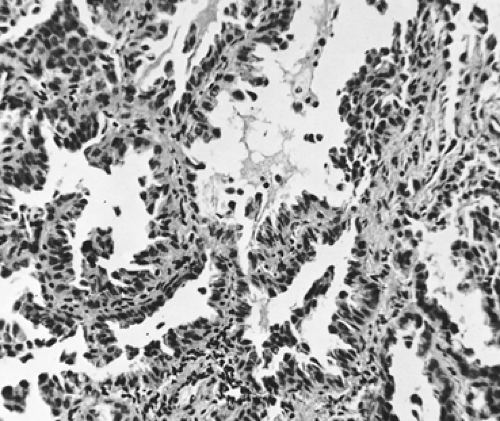
Adenocarcinomas may be divided into five major subtypes according to their growth pattern. These are (a) acinar and glandular, (b) papillary, (c) bronchioloalveolar carcinoma (BAC), (d) solid adenocarcinoma with mucin formation, and (e) adenocarcinoma with mixed cell types. Several minor variants, such as mucinous cystadenocarcinoma and signet-ring adenocarcinoma, have been described. The fetal adenocarcinoma is considered a major variant. However, not all investigators agree that the aforementioned subtypes are of necessary importance. Moori173 believes that only the bronchioloalveolar variant needs to be considered separately, but the fetal adenocarcinoma is discussed here as well. Noguchi and colleagues186 have suggested that small adenocarcinomas should be separated into two groups, each being differentiated into three distinctive structural patterns on the basis of the tumor’s growth patterns. The first group is composed of BAC tumors, in which the growth pattern involves the replacement of alveolar lining cells (“alveolar replacement group”), and the second is the “nonalveloar replacement group,” which is made up of typical adenocarcinomas (Table 104-3). Types A and B are considered to be in situ noninvasive peripheral adenocarcinomas and type C is believed to be an advanced stage of type A or B, which shows foci of active fibroblastic proliferation. Types D, E, and F are small, typical advanced adenocarcinomas. Miyoshi and associates168 have described a subtype of micropapillary pattern in small (early) adenocarcinomas. Histologically, in this subset there are no central fibroblastic cores of the sort normally seen in the usual early papillary tumors. The long-term survival of p (post-resection) stage I lesions without the presence of a central core versus that of the papillary lesions with a central fibrovascular core was 93% and 79% at 5 years and 89% and 69% at 10 years, respectively; both percentages being statistically significant.
Table 104-3 Modified Noguchi Classification of Small Adenocarcinomas | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
The usual glandular variety of adenocarcinoma arises in the peripheral area of the lung, although one-fourth or even more may occur in the central area. Many of the tumors are found to arise in areas of chronic interstitial fibrosis and in conjunction with a lung scar. The so-called scar carcinoma was presumed to arise from or in the scar tissue. The studies of Barsky and coworkers18 and Madri and Carter,149 however, suggested that the scars were secondary to the desmoplastic properties of the carcinoma, and it is now believed that these tumors do not represent an adenocarcinoma arising in a scar. Clinically, these so-called scar carcinomas behave as typical adenocarcinomas. It is of interest that the presence of a fibrotic focus (scar) in small peripheral adenocarcinomas is of prognostic significance. Noguchi186 and Eto66 and their associates, among others, noted that the prognosis was poorer when central fibrosis was present than when it was absent. Recently, Suzuki and coinvestigators262 have shown that the size of the central fibrosis is also a significant prognostic factor. When the central fibrosis was <5 mm, the prognosis was excellent; but as the size increased up to 15 mm, long-term survival was less. Furthermore, the worst prognosis was seen when the size of the fibrosis exceeded this 15-mm limit. In contrast to the poor prognosis of the increasing size of central fibrosis, the presence and increasing size of a ground-glass opacity on HRCT of adenocarcinomas ≤3 cm in size portends a better prognosis. The incidence of lymph node involvement is lessened or even absent when a greater percentage of the tumor has a ground-glass appearance. Matsuguma and coworkers,159 in a series of 96 patients with these small tumors, found that lymph node metastases were absent when >50% of the lesion (26 patients) had a ground-glass opacity on HRCT. When the percentage of the opacity was >50% (70 patients), 18.7% were
found to have lymph node involvement. Lymphatic and vascular invasion showed a similar correlation.
found to have lymph node involvement. Lymphatic and vascular invasion showed a similar correlation.
The growth rate of adenocarcinomas is intermediate between that of the squamous cell and the undifferentiated large-cell types. Peripheral adenocarcinomas are being found in increasing numbers as small nodules 5 to 15 mm in size by helical CT scans of the lungs in asymptomatic high-risk patients, as reported in the screening studies of Kaneko117,118 and Sone258 and their colleagues in Japan as well as by Henschke and Yankelewitz92 and Henschke and associates100 in the United States. When undetected, peripheral adenocarcinomas may enlarge significantly and yet remain asymptomatic. Of interest is that in contrast to peripheral squamous cell tumors, necrosis with cavitation is rarely observed in large peripheral adenocarcinomas. These tend to spread early by way of the vascular system. Lymphatic invasion and lymph node metastases are also common early in the course of the disease, and small adenocarcinomas appear to have a greater propensity to do so than similarly sized squamous cell tumors. Sagawa and associates241 reported an incidence of mediastinal node metastasis of 20% in patients with adenocarcinomas ≤3 cm in size as compared with a 10% incidence in similarly sized small squamous cell lesions. Sakurai and colleagues243 reported an incidence of lymph node involvement of 36% in adenocarcinomas (exclusive of BAC tumors) ≤3 cm in size.
In addition to the size of the adenocarcinoma, the location of the primary tumor in the lung, either central or peripheral (located in the outer two-thirds of the lung field) influences the incidence of lymph node metastasis. According to a study by Ketchedjan and associates122 of 514 adenocarcinomas (excluding BAC tumors), 111 were located in the central area and 403 were located in the peripheral area. The incidence of lymph node metastasis was greater when the cancer was located in the central area. The authors presented the data relative to T1 tumors in both groups. In 24 patients with T1 tumors located in the central region, the incidence of nodal involvement was 50% regardless of tumor size (between 0.1 to 1, 1.1 to 2, and 2.1 to 3 cm). In the 267 patients with peripheral T1 tumors, the incidence of nodal involvement was 23.9%. Peripheral T1 adenocarcinomas between 0.1 and 2 cm in size had an incidence of lymph node involvement of 17.2%; those between 2.1 and 3 cm in size had an incidence of 34%. Data for the other tumors were not given.
Microscopic Features
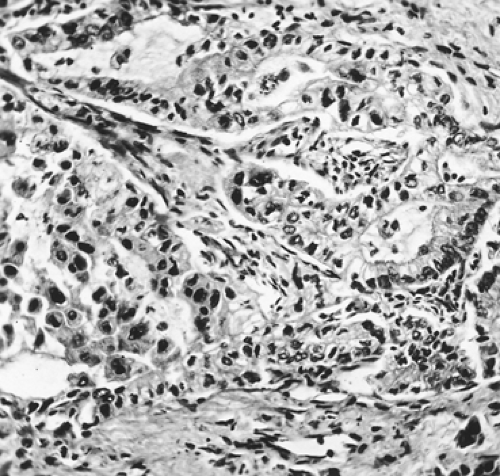
Well-differentiated adenocarcinomas are composed of cuboidal to columnar epithelial cells with fairly uniform round nuclei; they have adequate pink or vacuolated cytoplasm, are arranged in distinct acinar or glandular patterns, and are supported by a fibrous stroma. The cells may show papillary intraluminal growth and may contain mucicarmine-positive vacuoles or secretions. Miyoshi and associates168 have described an early-stage lung adenocarcinoma with a micropapillary pattern without any evidence of a central fibrovascular core. Such tumors appear to have a worse prognosis than the aforementioned cell types. These authors observed a statistically significant decrease in survival of 79% and 67% at 5 and 10 years when tumors of this type were present as compared with other early lesions without this cellular pattern, which were associated with survivals of 93% and 87% at 5 and 10 years. Moderately differentiated tumors are composed of nests, cords, or isolated cells, occasionally arranged in an acinar or glandular pattern (Fig. 104-3). The cytoplasm is supported by a fibrous or desmoplastic stroma. Mucicarmine-positive vacuoles may be present in this histologic subtype as well. Poorly differentiated tumors are composed predominantly of anaplastic cells of variable size and shape, with minimal but distinct evidence of acinar formation. Mucicarmine-positive vacuoles may be present. It has been suggested that tumors without distinct acinar formation should not be placed in the category of adenocarcinomas.
Electron Microscopic Features
Adenocarcinomas are composed of cuboidal or columnar cells. Aggregates of chromatin are dispersed inside the nuclei. Well-developed apparatuses are often missing or poorly formed in many of the tumor cells. Intracellular or intercellular lumina are present. Mucin production is evident, and Clara cell or type II pneumocyte differentiation may be identified. The tumor cells show the presence of straight microvilli. Desmosomes and terminal bars may be identified.
Immunohistochemical Features
According to Lee and associates142,143 and as summarized by Gould and Warren,81 adenocarcinomas of the lung are readily immunostained with monoclonal antibodies that recognize either a membrane-associated glycoprotein molecule or the specific sugar sequence found in lacto-N-fucopentose III. Of particular interest is that these reactions, according to Gould and Warren,82 are either absent or weakly expressed in diffuse mesotheliomas and thus may be a differentiating feature when a mesothelioma presents histologically as a glandular, papillary structure. Adenocarcinomas also immunostain for epithelial keratins CK7, 8, 18, and 19. According to van de Molengraft and associates,278 CK7 antibody (OV-TL 12/30) is a marker for glandular differentiation in lung cancer. CK14, found in all squamous cell tumors, is not present. Asada and colleagues9 have
noted further that in most adenocarcinomas (26 of 29 tumors in the study), dipeptidyl aminopeptidase IV activity is expressed. Other markers may be identified, including CEA, found in approximately 75% of adenocarcinomas. Epithelial membrane antigen, leu-7, and vimentin are found in a minority of tumors. Surfactant apoproteins (PE-10 immunoreactivity or Clara cell protein) may be demonstrated in approximately 50% of tumors, according to Mizutani and colleagues.169 The latter investigators did not identify PE-10 activity by pulmonary tumors of other cell types; however, Nicholson and associates96 have described the presence of PE-10 reactivity in both small-cell carcinomas and atypical carcinoids. Last, neuroendocrine differentiation features can be identified by staining techniques using monoclonal antibodies. Hiroshima and colleagues97 reported that neuroendocrine differentiation was identified in 23% (21 tumors) of 90 resected adenocarcinomas ≤3 cm in size. Only one-third of these (7 tumors, 7.8% of the total number of specimens) had, by the authors’ criteria, sufficient neuroendocrine differentiation to presage a poorer prognosis than that of patients with tumors with no (76.7%) or nonsufficient neuroendocrine features (15.6%). The 5-year survivals were 57% for the first group and approximately 93% for the latter two groups, respectively. As with squamous cell carcinomas, other multiple molecular markers may be identified, as documented by D’Amico and colleagues50 as well as numerous other investigators.
noted further that in most adenocarcinomas (26 of 29 tumors in the study), dipeptidyl aminopeptidase IV activity is expressed. Other markers may be identified, including CEA, found in approximately 75% of adenocarcinomas. Epithelial membrane antigen, leu-7, and vimentin are found in a minority of tumors. Surfactant apoproteins (PE-10 immunoreactivity or Clara cell protein) may be demonstrated in approximately 50% of tumors, according to Mizutani and colleagues.169 The latter investigators did not identify PE-10 activity by pulmonary tumors of other cell types; however, Nicholson and associates96 have described the presence of PE-10 reactivity in both small-cell carcinomas and atypical carcinoids. Last, neuroendocrine differentiation features can be identified by staining techniques using monoclonal antibodies. Hiroshima and colleagues97 reported that neuroendocrine differentiation was identified in 23% (21 tumors) of 90 resected adenocarcinomas ≤3 cm in size. Only one-third of these (7 tumors, 7.8% of the total number of specimens) had, by the authors’ criteria, sufficient neuroendocrine differentiation to presage a poorer prognosis than that of patients with tumors with no (76.7%) or nonsufficient neuroendocrine features (15.6%). The 5-year survivals were 57% for the first group and approximately 93% for the latter two groups, respectively. As with squamous cell carcinomas, other multiple molecular markers may be identified, as documented by D’Amico and colleagues50 as well as numerous other investigators.
Fetal Adenocarcinoma
Well-differentiated fetal adenocarcinomas of the lung (WDFA) have a histologic resemblance to the epithelial tubules of the embryonic lung in the first trimester of pregnancy (10 to 16 weeks of gestation), as initially described by Kradin and associates134 under the name of pulmonary endodermal tumor resembling fetal lung (PET). According to Tomashefski,270 fetal adenocarcinomas constitute a heterogenous group of adenocarcinomas ranging from WDFA to more poorly differentiated, more aggressive PETs. Kodama and colleagues128 discussed the former and Mardini and coworkers153 described one of the latter variants: an adenocarcinoma with endometrioid features and prominent neuroendocrine differentiation.
Larsen and Sorensen139 described 23 cases of WDFA. The gender distribution was approximately equal, with a median age of 40 years and an age range of 12 to 73 years. An earlier series of WDFAs reported by Koss and associates132 was slightly different in gender distribution and age limits but neither was of great significance. Most patients are or were smokers. Approximately 40% of the patients are symptomatic, with various respiratory complaints.
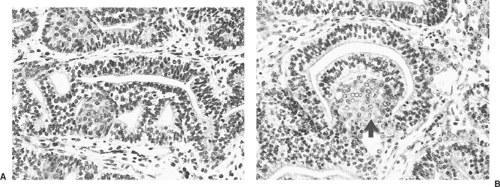
The size of the tumor may vary greatly (1 cm to >10 cm); radiographically, most are unilateral pulmonary masses randomly located in the lung. Tomashefski270 notes that approximately 21% of the tumor protrudes into the bronchial lumen. Nakatani and associates177 standardized the description of the histologic features of WDFA in five cases. The neoplastic glands are typically lined by pseudostratified columnar epithelium with uniform oval nuclei and focally clear cytoplasm (Fig. 104-4A). Characteristic morules, described by Colby and colleagues, (1995) consist of a solid nest of cells beneath the glandular epithelium with scant benign stroma (Fig. 104-4B). Immunohistochemically, the endothelial cells are positive for keratin, CEA, and epithelial membrane antigen (EMA). Vimentin may or may not be identified. The stromal spindle cells are positive for vimentin and occasionally for muscle-specific actin and desmin. Neuroendocrine cells may be present, and these stain for a number of polypeptide hormones.
The poorly differentiated (atypical, aggressive) fetal adenocarcinomas described by Kodama and coworkers128 have a poor prognosis. These tumors are larger and necrosis is abundant. Histologically, the tumors resemble the WDFA in its pattern, but the cells are columnar with clear, glycogen-rich cytoplasm, atypical nuclei, and frequent mitoses are present. Nests of neuroendocrine cells may be present, as reported by Mardini and
associates.153 Stromal cells are sparse. The prognosis is much poorer for patients with atypical fetal adenocarcinoma than those with WDFA, as recorded by Nakatani and colleagues.178
associates.153 Stromal cells are sparse. The prognosis is much poorer for patients with atypical fetal adenocarcinoma than those with WDFA, as recorded by Nakatani and colleagues.178
Bronchioloalveolar Carcinoma
Bronchioloalveolar carcinoma (BAC) represents highly differentiated adenocarcinoma, but many pathologists have considered it a separate and distinct tumor. Nonetheless Moori,173 Noguchi,186 and Kondo129 and their associates, among others, believe that these tumors represent a unique subset of adenocarcinoma. They constitute 1.5% to 7.0% of all bronchial carcinomas, with an average incidence of 2.5%. However, Barsky and colleagues16 have noted an increasing incidence of these tumors; in 1955, the bronchioloalveolar carcinomas accounted for only 5% of resected lung cancer specimens, whereas in 1990, these tumors made up 25% of resected lung cancers.
Singh and associates257 described the criteria for the diagnosis of bronchioloalveolar carcinoma as follows: (a) absence of primary adenocarcinoma elsewhere in the body, (b) absence of a central bronchial adenocarcinoma, (c) a peripheral location, (d) growth using the alveolar septa as supporting structures (lepidic growth), and (e) a characteristic histologic appearance different from that of other lung tumors. Last, aerogenous spread of the tumor within the lung is believed to be important.
Microscopically, the alveolar spaces are lined by malignant cuboidal or nonciliated columnar epithelium in layers or in papillary formation (Fig. 104-5). The alveolar spaces may be filled or even distended with this proliferating epithelium. Occasionally, single cells or clusters containing large multinucleated giant cells may appear lying free. The nuclei are hypochromatic, but mitoses are not common. The cytoplasm is acidophilic and abundant, or the cells may be mucous cells with extensive amounts of mucus filling the alveolar spaces. The lung architecture is most often preserved, although a few specimens show diffuse malignant invasion. Scar formation is seen in approximately half the tumors. Also in approximately half the tumors, mucin-producing cells are observed as the dominant cell type. Clayton42 initially separated bronchioloalveolar carcinomas into two cell types: mucinous and nonmucinous (i.e., Clara cells and type II pneumocytes). Rarely, the tumor may consist of a mixture of the mucinous and nonmucinous subtypes. Sakurai and coworkers243,244 reported that BAC tumors <3 cm in size were nonmucinous in 84%, mucinous in 8%, and combined in 8% of cases, respectively.
Clayton43 has divided the bronchioloalveolar carcinomas into three subgroups. Histologically, the first group comprises tumors made up of high columnar, mucin-producing cells with little nuclear atypia: in Clayton’s series this group made up approximately one-fourth of all bronchioloalveolar tumors. This subtype tends to spread aerogenously, forming satellite tumors. The second subgroup is made up of tumors composed of cuboidal or low columnar cells with a high degree of atypia producing little or no mucin; psammoma bodies may be present. The third subgroup is formed by those tumors that have a central area of necrosis and often termed sclerosing bronchioloalveolar tumors. Barsky and associates16 reported the incidence of the three subtypes as nonmucinous in 48%, mucinous in 42%, and sclerotic in 10%, respectively. Of possible prognostic significance was that areas of dedifferentiation of BAC into solid areas of moderately or poorly differentiated adenocarcinoma were seen in 10%, 27%, and 42% of the nonmucinous, mucinous, and sclerotic types, respectively.
Clayton42 had earlier shown by electron microscopy that the bronchioloalveolar carcinomas may be composed of Clara-type cells, type 2 granular pneumocytes, or mucous cells. Barsky and colleagues17 also identified these three phenotypes in the bronchioloalveolar tumors and have suggested that these findings, as well as the multifocality of these cancers, are evidence of their multiclonal origin. This conclusion was supported to some extent by the study of p53 mutations in primary and secondary lung cancers by Mitsudomi and associates.166 However, Holst and coworkers100 studied eight multifocal cases of bronchioloalveolar carcinoma by using topographic genotyping, which enabled them to define the spectrum of point mutational changes in K-ras-2 and p53 oncogenes. They demonstrated that, although no commonality existed between the two gene mutations, the primary tumor and satellite and intrathoracic metastases showed the same point mutations of either oncogene when present. The authors concluded that their findings support a monoclonal origin of multifocal bronchioloalveolar carcinoma, the multifocal disease being the result of intra-alveolar, lymphatic, and aerosol spread.
Immunohistochemical studies by Lee and colleagues143 have shown that antibodies used to detect exocrine features that depend on sugar or glycolipid epitopes immunostain alveolar carcinomas regardless of mucin production. These tumor cells also immunostain for high- and low-molecular-weight keratin, epithelial membrane antigen, CEA, and leu-7.
In patients with peripheral adenocarcinomas <20 mm in size, Kondo and associates129 identified 102 (84.2%) BAC tumors (aveolar replacement growth), most of which were either type A or B; less than one-third were type C, the more aggressive variety. No lymph node metastases were noted in any patients of this entire group, although metastasis may occur with type C tumors. The 19 (18.6%) patients with type D, E, or F tumors (true adenocarcinoma—nonalveolar replacement growth) likewise had no metastatic node involvement, but lymphatic vessel invasion was noted in three patients.
Grossly, BAC tumors may occur in one of three forms: solitary, multinodular, and diffuse or pneumonic type. The first is the most common and accounts for slightly less than half to almost two-thirds of the tumors of this subclassification. In the series reported by Hill,95 the percentage of a solitary mass or nodule was 43%; in the series reported by Harpole,87 Daly,54 and Ebright64 and their colleagues, the percentages were 59%, 69%, and 64%, respectively. Approximately 66% of the solitary tumors in the series of Daly and associates54 were ≤3 cm in diameter. Only a small number of these, however, fit the criterion of a solitary pulmonary nodule (i.e., a well-demarcated lesion <3 cm in diameter completely surrounded by pulmonary parenchyma). In the aforementioned series of 184 bronchioloalveolar carcinomas, only an 11.2% incidence of “solitary pulmonary nodules” was recorded. The vast majority of the small lesions may be ill-defined infiltrates, and an increasing number of these are being identified by HRCT. The larger, diffuse tumors may appear as masses, as infiltrates, or even consolidations of an entire lobe. The multinodular, unilateral, or bilateral lesions make up just between 10% and 20% of the total. Direct extension beyond the lung is uncommon and lymph node metastasis is likewise infrequent and, as noted in most series, do not occur with types A and B tumors. Daly and associates54 recorded only a 7.5% incidence of lymph node metastasis in tumors of the C type. Ebright and coworkers64 reported a similar incidence (7.2%) in the series from the Memorial Sloan-Kettering Cancer Center. The classification of BAC tumors by Ebright and associates64 is different than that of Noguchi and colleagues.186 The former authors64 separate BAC tumors into three groups: (a) pure bronchioloalveolar carcinoma, (b) bronchioloalveolar with local invasion, and (c) adenocarcinoma with bronchioloalveolar carcinoma features. The incidence of each of these in their 100 patients was 47%, 21%, and 32%, respectively. Although their data and conclusions are convincing, only those data relative to types (a) (pure BAC) and (b) (BAC with local invasion) are discussed here. Of these 68 cases, 60% were unilocal lesions, 29.4% were multifocal (uni- or bilateral), and 10.2% were pneumonic in nature. The median survival was not reached at the time of publication for pure BAC tumors, whereas it was 52.2 months for the multifocal lesions (for the bilateral multifocal cases it was 75 months); it was only 18.7 months for the pneumonic type. Only four patients developed lymph node metastases, and distant metastases were seen in six cases (the brain and skeletal system being the more common sites). However, local recurrence (locoregional or a distant metastasis) and the appearance of a new primary were common; in pure BAC patients the incidence was 51.2%; 57.1% local recurrence and 42.8% distant spread of the tumor in the thorax. New primary lesions were noted in 18.7%. For patients with BAC with local invasion, the overall incidence was 50% and these cases were equally divided between recurrent disease and new primaries. Of the seven patients with a pneumonic lesion, two had relapses and three developed new cancers. No lymph node metastasis was identified, although one patient died of a brain metastasis.
Significance of Bronchioloalveolar Carcinoma
With the increased identification of small peripheral nodules <2 cm in size by helical CT and subsequent HRCT, the separation of atypical adenomatous hyperplasia (AAH), bronchioloalveolar carcinoma (BAC), and frank adenocarcinoma has become very important, since the management and prognosis of each of these lesions are markedly different. The differentiation of AAH and BAC has been discussed above. Thus the current focus is on the separation of BAC from frank adenocarcinoma. Noguchi and associates,186 as noted, have separated these latter two subtypes into six categories by using a relatively simple histologic classification. These are (a) type A, localized bronchioloalveolar carcinoma with replacement growth of alveolar-lining epithelial cells; (b) type B, localized bronchioloalveolar carcinoma with foci of structural collapse of alveoli; (c) type C, type A with foci of active fibroblastic proliferation; (d) type D, poorly differentiated adenocarcinoma; (e) type E, tubular adenocarcinoma; and (f) type F, papillary adenocarcinoma with a compressive growth pattern. Types A and B have no or only rare associated lymph node metastases and have the most favorable prognosis. Small peripheral (≤20 mm) lesions have a 5-year survival rate of 100% after resection. These observations were confirmed by Wu295 and Sakurai243,244 and their coworkers. Furthermore, Brethnach and associates,30 in a review of 33 patients with stage I bronchioloalveolar carcinoma (the inclusion of type C must be assumed) and 105 patients with stage I adenocarcinoma (see Chapter 109 for staging criteria), reported a 5-year survival of 83% for the former and 63% for the latter patients, respectively, the difference being statistically significant.
Undifferentiated Large-Cell Carcinoma
Gross Features
Because of the lack of uniformity in the criteria for the histologic diagnosis of undifferentiated large-cell carcinoma (ULCC), their actual incidence is unknown, but it is probably between the 4.5% recorded by Shinton255 and the 15.0% recorded by Yesner and associates.300 Travis and associates275 reported the incidence to be 9%. These tumors may occur in either the central or the peripheral zone, although the latter site is somewhat more common. They spread earlier and have a relatively poorer prognosis than that associated with the more differentiated non-small-cell types. The peripherally located tumors may cavitate, but the incidence is less than that seen in peripheral squamous cell tumors (6% versus 15% to 20%, respectively).
Microscopic Features
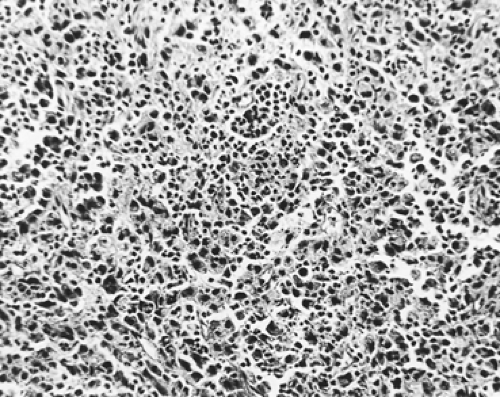
These heterogeneous tumors, which cannot be classified readily either as squamous cell carcinomas or as adenocarcinomas, are considered anaplastic tumors that show no apparent evidence of squamous or glandular differentiation (Fig. 104-6). Tumors composed of stratifying cells without evidence of intercellular bridge formation or keratin production are included in this group. Individual cells have enlarged, irregular vesicular or hyperchromatic nuclei that may have prominent nucleoli. The cells have abundant cytoplasm and may show a high mitotic rate.
Electron Microscopic and Immunohistochemical Features
Undifferentiated large-cell carcinomas show marked variation in cell shape. Cell membranes are often indistinct. Large nuclei with abundant cytoplasm and numerous organelles are present. Occasionally, tonofilaments are identified, but no distinctive features are present to permit categoric determination of cell type.
Warren and associates285 noted that dense-core granules and predominantly neuroendocrine differentiation appear in 40% of the so-called large-cell tumors; such tumors are now classified as large-cell neuroendocrine carcinomas.
Warren and associates285 noted that dense-core granules and predominantly neuroendocrine differentiation appear in 40% of the so-called large-cell tumors; such tumors are now classified as large-cell neuroendocrine carcinomas.
Piehl and associates217 suggested that approximately 50% of undifferentiated large cell carcinomas could be regarded as poorly differentiated adenocarcinomas, given their expression of exocrine phenotype antigens. Also, Gould and colleagues78 reported that most of these large-cell undifferentiated carcinomas express cytokeratin polypeptides of the simple epithelial type shown by adenocarcinomas and neuroendocrine neoplasms and only a small number display cytokeratin of the epidermal type that is characteristic of squamous cell car- cinoma.
Clear Cell Carcinoma.
Clear cell carcinoma is considered a subtype of large-cell anaplastic lung cancer, although it can also be a variant of either a squamous cell tumor or an adenocarcinoma. Histologically, these tumors are composed of malignant cells in nests, sheets, or clusters with large vesicular nuclei and abundant clear cytoplasm, which may or may not contain glycogen. Edwards and Carlyle65 reviewed six cases of clear cell lung carcinoma. On further analysis of the tissue by both light and electron microscopy, they found glandular differentiation in three cases and squamous differentiation in two. They concluded that clear cell carcinoma should not be considered a separate entity. Katzenstein and colleagues119 reviewed 348 cases of lung cancer and found 15 tumors with clear cells; 10 of these showed foci of epidermoid differentiation and 4 had glandular differentiation. Only one lesion qualified as a true clear cell carcinoma. In summary, these two reports question the validity of having a separate category of clear cell anaplastic carcinoma, given that these tumors really appear to be poorly differentiated squamous cell carcinomas or adenocarcinomas. Even when one is attempting to classify a lesion as a clear cell carcinoma, other pathologic diagnostic possibilities must be considered (Table 104-4). Gaffey and coworkers73 note that immunohistochemical and electron microscopic features are necessary to establish the proper diagnosis. The features of the benign clear cell tumor (sugar tumor) are discussed in detail in Chapter 125, especially its uniqueness in its reactivity to HMB45, a melanogenesis-related marker.
Table 104-4 Clear Cell Tumors of the Lung | ||
|---|---|---|
|
Adenosquamous Carcinoma
Adenosquamous carcinomas are defined as tumors composed of an admixture of squamous cell carcinoma and adenocarcinoma. At least 5% of the tumor should be composed of the minority cell type, according to the criteria of Takamori263 and Shimizu253 and their coworkers, although the World Health Organization (1982) gives no reference to the actual ratio. The Japan Lung Cancer Society115 has stated that at least 20% of the tumor should be formed by the less dominant variety; this group also notes that the tumors should be categorized as 20% to 40%, 40% to 60%, and 60% to 80% of either one of the cell types. The overall incidence of adenosquamous carcinoma is between 0.4% and 4.0%; in the surgical series reported by Takamori263 and Shimizu253 and their associates, the incidence was 2.6% and 3.4%, respectively. These tumors are more often peripheral rather than central in location. Although Ishida and coworkers106 reported an almost equal occurrence in men and women, Shimizu and associates,253 in a much larger series (44 patients versus 11 patients), reported a marked preponderance of men (35:9). Three-fourths of these tumors were >3 cm in size, with a range of 1.5 to 6.0 cm. Microscopically, either component may be well, moderately, or poorly differentiated. The squamous cell component was the dominant tumor in the majority of cases in Shimizu and associates’253 report.
Ichinose and associates102 reported that the DNA ploidy patterns of both components showed that, despite the different phenotypes, in 67% of the specimens examined, similar biological characteristics were present. These findings support the theory of Steele and Nettesheim278 that these tumors arise from instability of differentiation. Immunohistochemically, pankeratin stained both the squamous and glandular components. The high-molecular-weight keratin stained mostly the squamous component and not the glandular one. The low-molecular-weight keratin stained the glandular component slightly more often than the squamous component, but in most cases, it
did not stain either component to any great extent. CEA and epithelial membrane antigen stained both elements of most tumors with relatively high frequency.
did not stain either component to any great extent. CEA and epithelial membrane antigen stained both elements of most tumors with relatively high frequency.
Table 104-5 Comparison of Histologic Types Between the Primary Adenosquamous Lesions of the Lung and Lymph Node Metastasis | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||
Blood vessel invasion is common, and lymph node metastasis occurs in over 50% of patients. The latter was present in 61% of the specimens in Takamori and associates’263 series. The incidence of lymphatic metastasis appears to be greater in patients with a higher percentage of adenocarcinoma present in the tumor (Table 104-5). The cell type of the metastatic disease can vary. Overall, the prognosis is poorer than that of either a squamous cell carcinoma or adenocarcinoma of a similar stage.
The aforementioned impression of the poor prognosis of adenosquamous carcinoma of the lung has been further supported by the observations of Nakagawa and coworkers.176 The surgical results in 30 patients (2.4%) compared with the results in 1,219 cases of either squamous cell or adenocarcinoma treated during the same period showed a cumulative survival rate of 6.2% at 5 years versus one of 41.5%, respectively. The 5-year survival rate of stage I and II (18 patients) was only 9.7% for those patients with adenosquamous cell carcinoma and 55.4% (771 patients) with either squamous cell or adenocarcinoma of the same stages. Furthermore these authors noted that a greater number of patients with adenosquamous cell carcinoma have a higher stage of disease when it is initially identified. The studies of the aforementioned authors, in addition to those of Fitzgibbons and Kern69 as well as Naunheim183 and Riquet230 and their colleagues, support these conclusions. It should be noted, however, that Sridhar and associates259 found that there was no major difference in the survival rate of patients with resected local stage disease, albeit a small percentage (10%) of the patients with adenosquamous cell carcinoma and those with other, similarly staged NSCLCs, especially those with adenocarcinoma or large-cell tumor.
Basaloid Carcinoma
Basaloid tumors of the lung are uncommon lesions. They are considered a variety of the non-small-cell, nonneuroendocrine lung carcinomas. Prior to the initial description of this tumor by Brambilla and associates,28 such tumors were considered to be either a poorly differentiated squamous cell tumor or a poorly differentiated large-cell tumor. In fact, in the World Health Organization (WHO) classification,268 the basaloid tumor is listed in both the squamous cell and large-cell categories. Nonetheless, the basaloid tumor is now considered to be a separate entity.
Grossly these tumors are located in a lobar or segmental bronchus in 85% of the cases; 15% are located more distally. The tumor is usually exophytic but is associated with bronchial wall invasion. Brambilla and associates27,28,29 have described the histological features of this tumor: small, moderately pleomorphic, cuboidal to fusiform cells with hyperchromatic nuclei, graular dense chromatin, and a scanty amount of cytoplasm. The mitotic index is high. Immunohistochemically, the basaloid carcinomas express low-molecular-weight cytokeratins. One of three neuroendocrine markers (discussed subsequently) may be present in up to 40% of the tumors, as in the study by Kim and colleagues123 of 24 patients with a pure form and 11 with a mixed form of the tumor. Sturm and coworkers261 reported that the presence of the thyroid transcription factor 1(TTF-1) excluded the diagnosis of basaloid carcinoma. Immunohistochemical staining for 34 β E12 (low-molecular-weight cyto- keratins) was present in 80% of the basaloid cancers. In Kim and colleagues’123 study, 34 β E12 was also present in 80% of patients and TTF-1 was negative in all. Genetic studies have shown that p53 is stabilized in 85% of the basaloid tumors. These tumors are thought to originate from the basal, pluripotent reserve cells of the bronchial mucosa. The major histologic differential diagnoses are a small-cell carcinoma, as discussed by Foroulis and associates,72 and large-cell neuroendocrine carcinoma, as noted by Kim and colleagues.123
Basaloid carcinomas of the lung have been considered to be highly aggressive, and despite resection of even early-stage lesions with or without subsequent irradiation, the 5-year survival rate reported by Moro and associates174 was only 15%. However, Kim and colleagues123 reported a 5-year survival rate of 57.2% after resection of stage I and II disease. This compared favorably with a 5-year survival of 53.9% in 85 stage I and II patients with resected poorly differentiated squamous cell carcinoma. Whether other series will substantiate these results remains to be seen.
Tumor Size of Non-Small-Cell Lung Cancer
It has become apparent that the size of NSCLCs is of greater importance than has been recognized in the past. This is particularly true of small, peripheral tumors ≤3 cm in size, but it is evident that a size of >3 cm is likewise relevant as to the pathologic behavior of a given tumor.
In an early series of patients with tumors ≤3 cm or less in size and without lymph node involvement Read224 and associates reported that the 5-year survival was significantly greater in the patients with tumors <2 cm in size as compared with those >2 to 3 cm in size. The reports of Port,219 Gajra,74 Okada,195,197 and Birim23 and their coworkers support the aforementioned significant findings (Table 104-6). Wisnivesky and colleagues,294 using a database* of over 7,000 patients with stage I disease, confirm these findings (Table 104-7).
Patz215 and Takeda264 and their associates, however, found no significant differences in similar groups of patients. In fact in
Takeda and coworkers’264 study, only a tumor size >5 cm was an independent significant prognostic factor. Subsequently Ohata and associates194 observed, in 86 patients with stage I NSCLC ≥5 cm in size, that 56.8% of the tumors relapsed within 12 months. The overall 5- and 10-year survival rates were 42% and 24.2% respectively. Watanabe290 and Carbone36 and their associates also reported that patients with tumors >5 cm in size had a worse survival rate than those patients with smaller tumors. However, in these latter two studies, tumors <2 cm in size had a significantly better prognosis than those >2 cm to 4 cm. Moreover, in an earlier report, López-Encuentia and colleagues,148 in a study of 1,020 patients with NSCLC, found the 3-cm cutoff not to be an appropriate prognostic threshold. Four other tumor sizes were better and stronger prognostic indicators: (a) 0 to 2 cm, (b) 2.1 to 4 cm, (c) 4.1 to 7 cm, and (d) >7 cm. Obviously more data are necessary to resolve this issue.
Takeda and coworkers’264 study, only a tumor size >5 cm was an independent significant prognostic factor. Subsequently Ohata and associates194 observed, in 86 patients with stage I NSCLC ≥5 cm in size, that 56.8% of the tumors relapsed within 12 months. The overall 5- and 10-year survival rates were 42% and 24.2% respectively. Watanabe290 and Carbone36 and their associates also reported that patients with tumors >5 cm in size had a worse survival rate than those patients with smaller tumors. However, in these latter two studies, tumors <2 cm in size had a significantly better prognosis than those >2 cm to 4 cm. Moreover, in an earlier report, López-Encuentia and colleagues,148 in a study of 1,020 patients with NSCLC, found the 3-cm cutoff not to be an appropriate prognostic threshold. Four other tumor sizes were better and stronger prognostic indicators: (a) 0 to 2 cm, (b) 2.1 to 4 cm, (c) 4.1 to 7 cm, and (d) >7 cm. Obviously more data are necessary to resolve this issue.
Table 104-6 Long-Term Survival Relative to Tumor Size in Millimeters | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
As to the two major cell types of small peripheral lung tumors, squamous cell carcinomas comprise only approximately 5% of such tumors, according to the study of Sakurai and colleagues.243,244 Of interest is that the number of peripheral lesions represented 22% of all squamous cell carcinomas resected by this group. Twenty-two squamous cell tumors ≤20 mm in size had a 13.6% incidence of lymph node metastasis, which is greater than the incidences of 6.3%, 7.4%, and 0% reported by Asamura,12 Oda,190 and Watanabe286 and their colleagues, respectively. In 33 patients with squamous cell tumors 20 to 30 mm in size reported by Sakurai and coworkers,244 the incidence of lymph node metastases was 33%. No skip metatases were seen in either group. The single tumor in the series <10 mm in size had no evidence of metastatic lymph node involvement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree