Pacing of the Diaphragm
Marina Feldman
John A. Elefteriades
History
Since the invention of the first electric generator by Otto von Guericke in 1663, investigators have tried to use electricity in medical experiments ranging from attempts to reverse paralysis to reanimation. The first to suggest electrical stimulation of the phrenic nerve in cardiopulmonary resuscitation was Carvalo,6 in his 1777 treatise on the uses of electricity for human ailments. Several years later, Hufeland published his doctoral dissertation, entitled: The Use of Electricity in Asphyxia. He recommended stimulation of the phrenic nerve to induce contractions of the diaphragm (Fig. 52-1).33 At the turn of the next century, Andrew Ure went further, demonstrating the electrical stimulation of the phrenic nerve of a “freshly hung criminal.” He reports, “The success of it was truly wonderful. Full … breathing instantly commenced. The chest heaved and fell; the belly was protruded and again collapsed, with the relaxing and retiring of the diaphragm.”61
The science of phrenic nerve stimulation was further advanced by Duchenne de Boulogne during the cholera epidemic of 1872,20 by Israel in 1927,36 and by Sarnoff in the 1950s52,53 in his work with polio victims. These early efforts were refined to permit long-term, continuous pacing of the diaphragm as it exists today through the pioneering work of Glenn and associates.
Continual advances in the art of electrophrenic respiration have allowed thousands of patients to enjoy longevity and a better quality of life.
Indications
There are currently two indications for the use of phrenic nerve stimulation: central alveolar hypoventilation and high cervical spinal cord injury.
Central Alveolar Hypoventilation
Central alveolar hypoventilation is a type of sleep apnea originating in a failure of respiratory drive with no specific primary defect in the neuromuscular or pulmonary systems per se. The brain fails to control the process of ventilation properly. This disease process stems from malfunction of the respiratory control center at the level of the medulla. The etiology of the disease is varied; it can stem from an infection (e.g., encephalitis), stroke, neoplasm, or trauma, including iatrogenic injury. Some cases, although thought to be idiopathic, probably represent dysfunction of the medullary chemoreceptors that normally detect hypoxia and hypercarbia.
The disease is categorized by blunted nocturnal response to hypoxia and hypercapnia with normal ventilation during waking hours. (There is, however, a wide spectrum of hypoventilation, ranging from only nocturnal hypoventilation to daytime hypoventilation as well.) The nocturnal preponderance underlies the eponym Ondine’s curse, used in the literature. Ondine was a water sprite who fell in love with a knight and married him. Once he became unfaithful to her, he was condemned to stay awake in order to breathe. If he fell asleep, he would forget to breathe and die.26 This legend has been a favorite of playwrights since 1811, and although the story has undergone many revisions and translations, the premise of nighttime hypoventilation is remarkably similar among different versions.
Patients with central alveolar hypoventilation often have an obese habitus. Chronic hypoxia and hypercapnia, as well as a fragmented sleep pattern, leads them to experience daytime somnolence. Over time, pulmonary vasoconstriction secondary to chronic hypoxia may lead to pulmonary hypertension and ultimately result in right-sided heart failure.
Another form of this syndrome, congenital central hypoventilation, has become increasingly recognized.23,35,60 The clinical presentation is an early deterioration in infants born with normal Apgar scores. The diagnosis necessitates exclusion of pulmonary and cardiac abnormalities as well as 24-hour respiratory monitoring.
The adequacy of the respiratory drive can be assessed by ventilatory response to hypercapnia and hypoxia (Fig. 52-2). Ventilation normally increases linearly with increasing PCO2 and increases as the partial pressure of oxygen (PO2) decreases. An impaired response to hypoxia or hypercarbia is seen in patients with central alveolar hypoventilation. An intermediate ventilatory response can be seen in individuals with primary weakness of the respiratory muscles. These conditions can mimic central hypoventilation; however, standard pulmonary function tests can readily distinguish between the two conditions. In diagnosing central alveolar hypoventilation, the authors routinely perform a 24-hour respiratory control study, with hourly assessment of asleep–awake status, end-tidal CO2, and O2 saturation. Patients with central hypoventilation demonstrate a characteristic pattern of severe nocturnal hypoventilation and hypercarbia with milder daytime hypercarbia.
Quadriplegia
The most common affliction that may be treated by diaphragmatic pacing is cervical spinal cord injury. With increased public awareness of cardiopulmonary resuscitation techniques, improved delivery systems for emergency care, widespread availability of positive-pressure ventilation, and improved long-term respiratory care, more patients are surviving the injury that caused their quadriplegia and are being sustained for longer periods than in earlier eras. Among the many spinal cord patients undergoing diaphragmatic pacing was the actor Christopher Reeve.
It is estimated that the annual incidence of spinal cord injury in accident survivors is approximately 40 cases per million population in the United States or approximately 11,000 new cases each year. The prevalence is estimated to be approximately 253,000 people. Motor vehicle crashes account for 46.9% of reported cases; the next most common cause is falls, followed by acts of violence (primarily gunshot wounds) and recreational sporting activities.7
Burney and colleagues have observed that the cervical region of the spinal cord is most commonly involved9; more than half of these injuries have been reported to result in quadriplegia or some degree of quadriparesis. In the adolescent population, sporting event accidents are also a common cause of cervical injury,47 while in the elderly population, cervical fractures often occur secondary to an accidental fall.59 Iatrogenic injuries may be seen as well. In addition to traumatic etiologies, infections, developmental and vascular abnormalities, and infarctions can also produce quadriplegia.
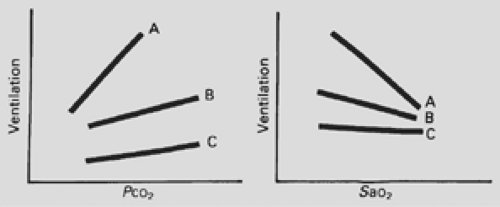 Figure 52-2. The ventilatory response to hypercapnia and hypoxia. A: Healthy controls. B: Patients with chest wall disorders. C: Patients with central hypoventilation. |
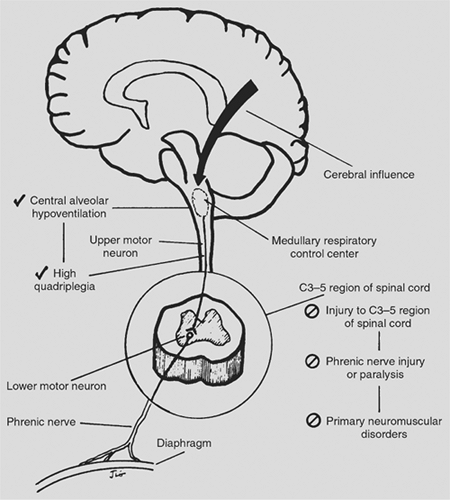
The lower motor neurons of the phrenic nerve are located in the spinal cord at the levels of C3 to C5. Spinal cord disruption below these levels causes quadriplegia but does not disrupt respiration. Spinal cord injury involving the C3 to C5 levels, where the cell bodies of the phrenic nerve are located, may partially injure a phrenic nerve and the patient may retain a limited ability to ventilate. However, ventilation may not be improved with the addition of diaphragmatic pacing because the phrenic nerve is compromised by degeneration. The decision to pace under these circumstances requires careful consideration, because the benefits may be limited.
High cervical quadriplegia, at or above C3 level, severs the tracts leading from the medullary respiratory control center to the spinal cord, preserving the integrity of the phrenic nerve (Fig. 52-3). In such patients, this makes diaphragmatic pacing possible.
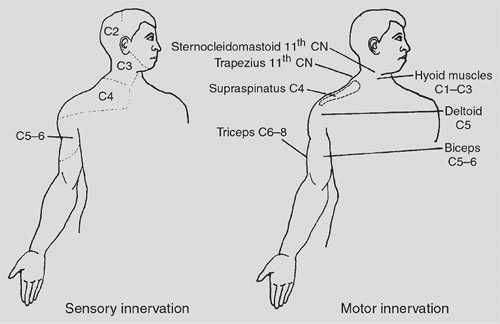
High cervical spinal cord lesions also denervate the intercostal (T1 to T12), abdominal (T7 to L1), and pelvic (L1 to S2) accessory muscles of respiration. Only the sternocleidomastoid and trapezius muscles, which are innervated by the spinal accessory nerve (cranial nerve XI), remain functional. In general, patients with high quadriplegia demonstrate intact sensation down to the clavicles; motor function is disrupted from the deltoid muscle and below (Fig. 52-4).
Intractable Hiccups
The use of diaphragmatic pacing has also been studied as a treatment for intractable hiccups. Pacing was found to be effective in treating patients with intractable hiccups2,25,49; however, the numbers of patients with truly intractable hiccups who require such surgical control are few.
End-Stage Chronic Obstructive Pulmonary Disease
As previously suggested by Glenn and colleagues, diaphragmatic pacing also has been used in patients with end-stage chronic obstructive pulmonary disease in whom ventilatory drive is based on hypoxia rather than hypercarbia.30 Administration of 24% oxygen can cause episodic respiratory failure in these patients because the hypoxic drive becomes increasingly diminished. Diaphragmatic pacing can provide ventilation independent of ambient oxygen levels. As with hiccups, the application of diaphragmatic pacing to patients with such severe chronic obstructive pulmonary disease has been limited.
Pacing Prerequisites
There are several criteria that must be met before instituting diaphragmatic pacing:
Severe condition of hypoventilation (e.g., central alveolar hypoventilation, high cervical quadriplegia)
Intact phrenic nerve
Acceptable intrinsic pulmonary function
Good diaphragmatic muscle function
Normal chest wall configuration
Satisfactory overall physical status
Absence of cognitive impairment
Prior to initiating diaphragmatic pacing, a severe hypoventilation condition must be documented.
Diaphragmatic pacing requires an intact phrenic nerve and a functional diaphragm. Figure 52-3 illustrates schematically the levels of neurologic insult correctable by diaphragmatic pacing. Phrenic nerve stimulation is not indicated for patients in whom the phrenic nerve itself is injured or dysfunctional. Examples include direct nerve injury from trauma, as a complication of cardiac surgery,12,17 or through involvement from malignancy or infection. Although successful phrenic nerve repair has been reported by Merav,43 the long-term merit of such endeavors remains unproven in terms of primary nerve function and ability to pace. There are efforts under way to bypass this requirement through transplant by replacing a dysfunctional phrenic nerve with vagal or intercostal branches. The preliminary forays into such nerve grafting have been reported by Krieger38,39 and by Baldissera and their colleagues.4 There are also efforts to combine intercostal and diaphragmatic pacing to improve ventilation.1,13,16 Experimental studies of direct intramuscular stimulation of the phrenic nerve have found limited clinical application.50,51 This technique is limited by the fact that, unlike the heart, the diaphragm is not an electrical syncytium; the pulse does not spread from cell to cell but rather depends on conduction via fine phrenic nerve radicals. Time and further experimentation will tell if damage to the phrenic nerve can successfully be corrected by medical science.
It is also critical that the diaphragmatic muscle be inherently sound in order to tolerate pacing. In general, a diaphragm affected by a primary muscular disorder such as myasthenia gravis or muscular dystrophy is not appropriate for pacing. Patients with central hypoventilation should demonstrate at least a 5-cm maximal excursion of the diaphragm with spontaneous breathing before embarking on diaphragmatic pacing; 8 to 10 cm is average. Quadriplegic patients are not required to exhibit the same degree of excursion with phrenic nerve stimulation as spontaneously ventilating patients. The diaphragm is expected to atrophy with disuse from quadriplegia and can be
rehabilitated with gradual conditioning. However, some brisk downward deflection of the diaphragm with percutaneous stimulation of the phrenic nerve should be observed, even in quadriplegia.
rehabilitated with gradual conditioning. However, some brisk downward deflection of the diaphragm with percutaneous stimulation of the phrenic nerve should be observed, even in quadriplegia.
There are efforts under way to create an artificial diaphragm.3 The Yale group is currently working with SRI using artificial muscle to create mechanical neodiaphragm.5
Diaphragmatic pacing requires that the ability of the lungs to oxygenate and ventilate be preserved. In most cases, pulmonary function test results should be normal or minimally compromised because pacing cannot compensate for severe restrictive or obstructive lung disease. For similar reasons, major deformities of the chest wall contraindicate diaphragmatic pacing by virtue of their mechanical interference with ventilatory function. Patients with congenital central hypoventilation syndrome become candidates for pacing after the age of 1 year, when the chest wall loses some of its pliancy.
Equally imperative for successful diaphragmatic pacing is a knowledgeable and cooperative patient. Patients who have suffered permanent cognitive impairment are not appropriate candidates for pacing. Paramount to the success of an individual patient is a supportive network of family members and friends and a professional team of health-care providers who are familiar with pacing techniques and the long-term care.
Phrenic Nerve Function
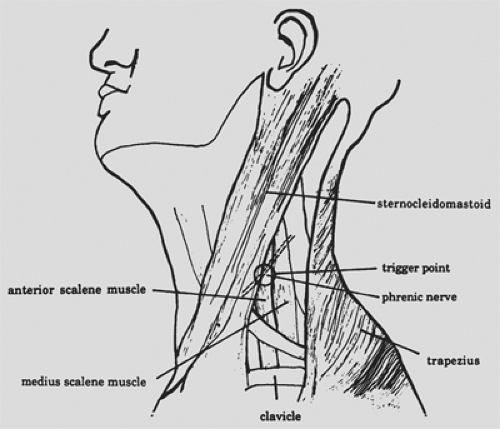
Because an intact phrenic nerve is essential for pacing, accurate assessment of nerve function assumes paramount importance. Sarnoff and Shaw53,56 have described the technique for transcutaneous testing of phrenic nerve conduction (Fig. 52-5). The overall technique is similar to that for any nerve conduction test. A thimble electrode facilitates testing of the phrenic nerve. The motor point of the nerve is located medial to the lateral edge of the clavicular head of the sternocleidomastoid muscle. With the sternocleidomastoid muscle retracted medially, stimulation is applied by the thimble electrode; the current is directed posteriorly. A brisk ipsilateral hemidiaphragmatic contraction should be readily visible and palpable on physical examination. Additionally, surface electrodes may be used to measure the action potential of the diaphragm and the phrenic nerve’s conduction time. Two electrodes are placed at the level of the eighth intercostal space, one in the anterior axillary line and the other in the posterior axillary line. A ground electrode is placed at the xiphoid. The phrenic nerve’s conduction time is measured as the elapsed time from phrenic nerve stimulation at the neck until a diaphragmatic action potential is recorded by an oscilloscope. The phrenic nerve’s conduction time in adults is normally 7.5 to 10.0 ms. A prolonged conduction time (greater than 11 ms) is considered abnormal; however, conduction times of 11 to 14 ms do not necessarily preclude pacing.
Phrenic nerve conduction time may also be assessed using cervical magnetic stimulation. A magnetic coil placed on the neck causes depolarization of the intraforaminal segments of cervical nerve roots C3 to C5. Bilateral, simultaneous stimulation of the nerve roots is usually performed; however, Mills44 has shown that unilateral stimulation is also possible. Because depolarization is applied to the intraforaminal segments of the phrenic nerve roots rather than to the body of the nerve, cross-stimulation of other muscle groups may occur. Careful interpretation of the cervical magnetic stimulation results is necessary. These results cannot be compared with those of percutaneous electromagnetic stimulation, in which the phrenic nerve is directly stimulated. Although Similowski and associates57,58 have reported on the usefulness of cervical magnetic stimulation for the selection of phrenic pacing candidates when used in combination with cortical magnetic stimulation, the number of patients evaluated is small, and application of this technique for phrenic nerve assessment is not standardized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree