Old nomenclature
New nomenclature
Femoral vein
Common femoral vein
Superficial femoral vein
Femoral vein
Sural veins
Sural veins; soleal veins; gastrocnemius veins (medial and lateral)
Huntarian perforator
Mid-thigh perforator
Cockett’s perforators
Paratibial perforator
Posterior tibial perforators
May’s perforator
Intergemellar perforator
Gastrocnemius point
The superficial venous system consists of a variable web-like plexus of veins, which are located superficial to the deep fascia and serve as a conduit to allow blood to pass centrally to the deep system, and eventually to the right atrium. The main superficial veins of the lower extremity are the great and short saphenous veins. The great saphenous vein (GSV) originates in the medial aspect of the dorsum of the foot and ascends anteriorly to the medial malleolus and becomes more posteromedially at the knee level and more medially at the thigh, where it ends by piercing the deep fascia at the cribriform fascia to join the common femoral vein at the saphenous-femoral junction (SFJ). The majority of patients have at least two major tributaries above the knee, the anterior and posterior circumflex. Three important pelvic tributaries join the GSV at the SFJ: the superficial inferior epigastric, the superficial external pudendal, and the superficial circumflex iliac veins. The clinical relevance of some of these veins will be discussed later. A duplicated main GSV is not uncommon, and many patients will have anterior or posterior accessory veins that run parallel to the main GSV.
The short saphenous vein (SSV) originates in the lateral aspect of the dorsum of the foot, passes posterolaterally to the Achilles tendon and runs posteriorly at midline superficial to the deep fascia, where it enters the popliteal fossa between the two heads of gastrocnemius. Most commonly, it joins the popliteal vein above the knee, but in some cases, it may join the GSV or deep muscular veins of the thigh.
The principal return of blood flow from the lower extremities is through the deep veins. In the calf, the deep veins are paired and named for the accompanying arteries. Therefore, the anterior tibial, posterior tibial, and peroneal artery are accompanied by their paired veins, which are interconnected. These join to form the popliteal vein, which may also be paired. As the popliteal vein ascends, it becomes the femoral vein [3]. Near the groin, this is joined by the deep femoral vein, which becomes the common femoral vein and then the external iliac vein proximal to the inguinal ligament.
The perforating veins are a communicating plexus of veins between the superficial and deep systems. They are named according to their location (Table 36.2). Understanding the anatomy of these veins will not only aid in determining the location of certain venous ulcers, but also in planning for therapy.
Table 36.2
Perforating veins (PV) of the leg
Gluteal perforators | Superior gluteal, midgluteal, lower gluteal PV |
Thigh perforators | Medial thigh PV (formerly Hunter’s perforator) |
(PV of the femoral canal or Inguinal PV) | |
Anterior thigh, lateral thigh PV, Posterior thigh PV (posteromedial, sciatic, posterolateral PV) Pudendal PV | |
Knee perforators | Medial knee PV (formerly Boyd’s perforator) Suprapatellar, lateral knee, infrapatellar, popliteal fossa PV |
Leg (calf) perforators | Paratibial, posterior tibial PV (formerly Cockett’s perforators) Anterior leg, lateral leg PV |
Posterior leg PV (medial and lateral gastrocnemius, Intergemellar, para-achillean PV) | |
Ankle perforators | Medial ankle, lateral ankle, anterior ankle PV |
Foot perforators | Dorsal foot or intercapitular PV |
Medial, lateral, plantar foot PV |
These veins penetrate anatomic layers, which gives them the name perforating veins. Veins that interconnect on the same anatomic layer are called communicating veins. In the leg, and using the now disfavored eponyms, the principal perforating veins have been named after an English surgeon, Frank Cockett. The Cockett I perforating vein is approximately 6 cm, measured from the floor in the standing patient. The Cockett II perforating vein clusters at about 12 cm, and the Cockett III is at 18 cm. These, and the 24 cm perforating vein, may need to be identified as tibial perforating veins in limbs with severe chronic venous insufficiency as they become targets for the surgeon. These perforating veins connect the posterior arch circulation to the posterior tibial veins.
In the upper anteromedial calf, a perforating vein is found and in the distal thigh, there are perforating veins. Above these in the mid-thigh, the perforating veins are named after John Hunter (Fig. 36.1).
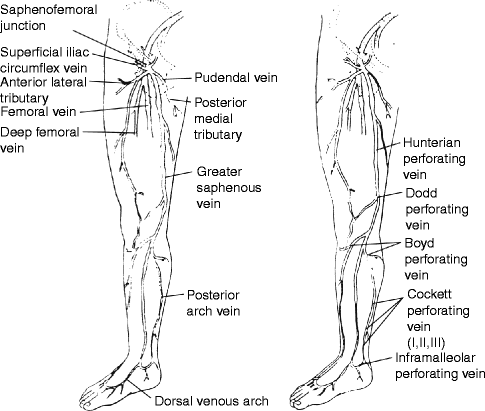

Fig. 36.1
The main superficial veins and perforating veins. Note that the Cockett perforating veins are part of the posterior arch circulation and that important tributaries to the saphenous vein at the groin include the anterolateral and posteromedial tributaries, either of which may simulate a duplication of the saphenous circulation
Normal Physiology of Venous Flow
Under normal conditions, about 90% of the venous return in the lower extremities is via deep veins, mainly facilitated through compartmental muscular contraction of the foot, calf, and thigh against tight fascia. The highest pressure is generated by the calf muscle and is estimated to be about 65%, in comparison to 15% generated by the thigh muscles [4]. As the muscle contracts, the pressure raises within the compartment which allows antegrade flow to the heart. In the meantime, valve closure prevents reverse venous flow or refluxing to the superficial system. As the muscle relaxes, the pressure within the compartment decreases and allows antegrade flow of venous blood from the superficial to the deep system (Figs. 36.2 and 36.3).
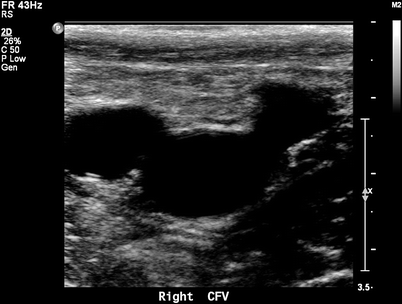
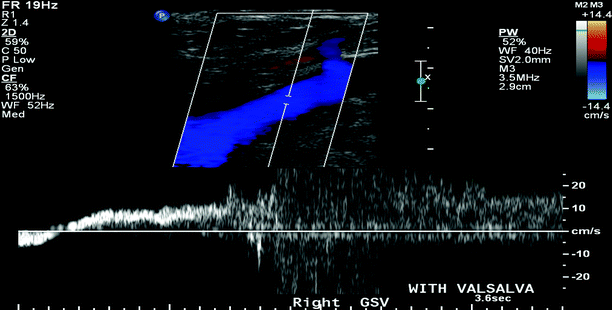

Fig. 36.2
B-mode duplex ultrasound for normal saphenofemoral junction showing the Mickey Mouse duplex sign, GSV, CFV, and CFA

Fig. 36.3
B-mode duplex ultrasound for significant reflux at the saphenofemoral junction elicited with Valsalva maneuver (>0.5 s)
There are many methods to classify venous diseases, depending on factors such as distribution and severity; however, the most effective method is to assess severity based on clinical, etiological, anatomical, and pathophysiological aspects. This international classification is not only an efficient description, but useful in determining appropriate management. Understanding the new terminology is crucial, not only to keep up with the American venous forum update, but also to serve as a common language among venous specialists.
Revised CEAP Classification of Venous Disorders
C: Clinical Findings
The letter “C” is based on clinical findings, usually seen on physical examination [5, 6]:
C0 = no visible venous disease
C1 = telangiectatic or reticular veins
C2 = varicose veins
C3 = edema
C4 = skin changes without ulceration
C5 = skin changes with healed ulceration
C6 = skin changes with active ulceration
More than one number may be assigned if the patient has several findings on clinical examination. After the numeric subscript, the letter “a” is assigned if the patient is asymptomatic or “s” if the patient experiences symptoms. Lastly, an additional number may follow the “s” to denote the severity of the symptom. The clinical disability scores for chronic venous insufficiency are:
0 = a patient who is asymptomatic, and thus has no disability
1 = a patient who is symptomatic, but can function without a support device
2 = a patient who can work an 8-h day with a support device
3 = a patient who is unable to work, even with a support device
E: Etiology
The “E” stands for etiology, with subscript “c” for congenital disease, “p” for primary disease (not due to another cause), or “s” for secondary venous disease, usually due to prior deep vein thrombosis.
A: Anatomic Findings
The “A” refers to the anatomic findings, usually based on duplex ultrasound examination. The options are as follows:
Superficial veins (As)
1.
Telangiectasias or reticular veins
2.
Great saphenous vein—above the knee
3.
Great saphenous vein—below the knee
4.
Small saphenous vein
5.
Nonsaphenous
Deep veins (Ad)
6.
Inferior vena cava
7.
Common iliac
8.
Internal iliac
9.
External iliac
10.
Pelvic: gonadal, broad ligament
11.
Common femoral
12.
Deep femoral
13.
Femoral (between the groin and the knee)
14.
Popliteal
15.
Crural: anterior tibial, posterior tibial, peroneal
16.
Muscular: gastrocnemius, soleus
Perforating veins (Ap)
17.
Thigh
18.
Calf
P: Pathophysiologic Component
The “P” refers to the pathophysiologic component, with subscript “r” for reflux, “o” for obstruction, or “r, o” for both reflux and obstruction.
There are multiple ways to understand venous disorders:
1.
Obstructive
(a)
Acute
Superficial
Deep
(b)
Chronic
Superficial
Deep
2.
Valvular dysfunction (Incompetence)
(a)
Superficial
(b)
Deep
(c)
Perforators
3.
Inflammatory
(a)
Superficial thrombophlebitis
4.
Congenital
(a)
May–Turner syndrome
(b)
Paget–Schrotter syndrome
(c)
Klippel–Trenaunay syndrome
Superficial Thrombophlebitis
Superficial thrombophlebitis (STP) is an inflammatory condition associated with thrombus formation within the superficial veins. It commonly occurs in the lower extremity; however, the incidence in the upper extremity is increasing, secondary to intravenous instrumentation. It usually has a benign and self-limited course. Clinical presentations include pain, redness, and swelling along the course of the affected vein. Fever and malaise are not uncommon. The pathophysiology can be explained in light of Virchows’s triad consisting of blood stasis, endothelial injury, and hypercoagulability. Therefore, a history of minor trauma or long travel may be the initial nidus for STP. Proper diagnosis and appropriate treatment is crucial to alleviate the pain and discomfort. A venous duplex scan will delineate the distribution of the STP and rule out the presence of DVT [7, 8]. The incidence of DVT is estimated to be as high as 40% in patients with STP. Migratory STP should raise the suspicion for underlying malignancy or other thrombophilias. Some diseases, such as Buerger’s disease, may be associated with migratory STP. Treatment is usually conservative and includes graduated compression stockings (30–40 mmHg), ambulation, and nonsteroidal antiinflammatory medications (Ibuprofen). Antibiotics are not recommended unless there is surrounding cellulitis. Special attention should be paid to a free-floating GSV thrombus at the SFJ, which should be treated with anticoagulation, or an IVC filter in those who have a contraindication to anticoagulation. Patients with iliofemoral DVT that meet the free-floating criteria are at significant risk for a pulmonary embolism, despite the administration of heparin [9].
Deep Venous Thrombosis (DVT)
Venous thromboembolism (VTE) is the fourth leading cause of death in Western countries. It is a common cause of morbidity and mortality. Although it is a preventable disease, the incidence of VTE exceeds 1/1,000 persons, with almost 400,000 new cases annually in the United States. Of patients who survive the initial event of VTE, 30% will have some kind of recurrence and 30% will develop postphlebitic syndrome within 10 years. Despite overwhelming evidence for the effectiveness of regimens [10–13], the concern over bedside prophylaxis and management often dissuades physicians from complying with guidelines.
The Virchow triad is often used to explain the development of perioperative DVT. Vascular stasis occurs primarily in the great veins of the body, which act as a reservoir system for circulation. At any given moment, approximately 70% of the total blood is contained in the venous system. Stagnation of venous blood is more likely when the patient is lying flat under the effect of anesthesia, particularly during laparoscopic surgery. The second component, hypercoagulability, occurs as a consequence of decreased clearance of the procoagulant factors (as seen in Table 36.3), or depletion of the lytic system (with or without underlying coagulopathies). The third component, endothelial damage, results from direct trauma to the vessel or by placement of a prosthetic graft. The combined influence of these factors promotes the development of venous thrombi in low-flow areas (e.g., subadjacent to the venous valves or adjacent to foci of intimal disruption). The propagation of thrombus leads to the development of overt DVT [14–20].
Table 36.3
Hypercoagulability factors
Thrombophilia | Normal | Incident VTE | Recurrent VTE | Incidence (95% CI) |
|---|---|---|---|---|
Factor V Leiden | 3–7 | 12–20 | 30–50 | 150 (80–260) |
Antithrombin III deficiency | 0.02–0.04 | 1–2 | 2–5 | 500 (320–730) |
Protein C deficiency | 0.02–0.05 | 2–5 | 5–10 | 310 (530–930) |
Protein S deficiency | 0.01–1 | 1–3 | 5–10 | 710 (530–930) |
Hyperhomocysteinemia | N/A | N/A | N/A | N/A |
Antiphospholipid Ab | N/A | N/A | N/A | N/A |
Prothrombin G20210A | 1–3 | 3–8 | 15–20 | 350 |
Combined thrombophilias | N/A | N/A | N/A | 840 (560–1,220) |
Factor VIII (>200 IU/dL) | N/A | N/A | N/A | N/A |
Leg swelling causes passive muscle engorgement and dilation of the muscle fibrils. These, in turn, activate pain receptors located in the muscle spindle and account for muscle discomfort. Stretching of the muscle aggravates the discomfort; this is the physiologic basis for the Homan’s sign (calf tenderness on dorsiflexion of the foot).
Additional factors that contribute to the pain are the inflammatory mediators released from stable clots that can contribute to the redness and leg swelling. DVT can be caused by numerous etiologies, including traumatic, vasculitic, hematologic, and drug-induced (Figs. 36.4 and 36.5).
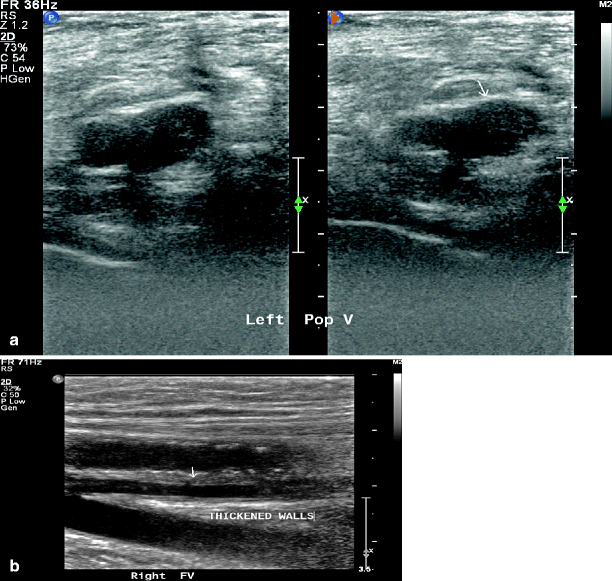
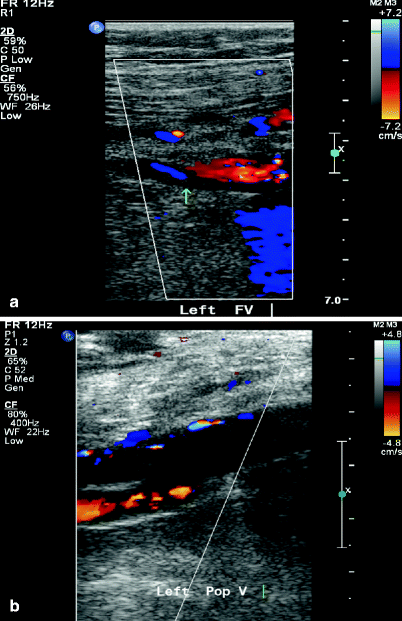

Fig. 36.4
Duplex ultrasound showing acute DVT of popliteal vein with both B-mode (arrow) (a) and colored duplex (arrow) (b)

Fig. 36.5
Duplex ultrasound showing chronic DVT of femoral vein with both B-mode (arrow) (a) and colored duplex (b)
Causes of DVT
General
Age, immobilization longer than 3 days, pregnancy and the postpartum period, major surgery in previous 4 weeks, long plane or car trips (>4 h) in previous 4 weeks
Medical
Cancer, previous DVT, stroke, acute myocardial infarction (AMI), congestive heart failure (CHF), sepsis, nephrotic syndrome, ulcerative colitis, multiple trauma, CNS/spinal cord injury, burns, lower extremity fractures, vasculitis, systemic lupus erythematosus (SLE) and the lupus anticoagulant, Behçet syndrome, homocystinuria
Hematologic
Polycythemia rubra vera, thrombocytosis, inherited disorders of coagulation/fibrinolysis, antithrombin III deficiency, protein C deficiency, protein S deficiency, Factor V Leiden, dysfibrinogenemias, and disorders of plasminogen activation
Drugs/medications
IV drug abuse, oral contraceptives, estrogens, heparin-induced thrombocytopenia
Pulmonary Embolism (PE)
Pulmonary embolism (PE) is one of the most dreaded complications of DVT. The true prevalence of perioperative PE is unknown; it varies according to the type of surgery, the use and type of prophylaxis, and the mode of diagnosis. Estimates indicate that without prophylaxis, fatal PE occurs in 0.1–0.8% of patients undergoing elective general surgery, 2–3% of those undergoing elective hip replacement, and up to 4–7% of those undergoing surgery for a fractured hip. The incidence of VTE increases after the age of 60 [10, 11].
Secondary to a potential lethal outcome of PE, early diagnosis is imperative for prompt therapy. Clinical presentation (shortness of breath, chest pain, and hemoptysis) and certain clinical criteria have been validated in the literature. The most commonly used validated criteria are the Wells score, which has been modified from year 1995 to 2001.
(a)
Previous history of PE or DVT—1.5 points
(b)
Clinical suspicion of DVT—3 points
(c)
Tachycardia—3 points
(d)
Alternative diagnosis is less likely than PE—3 points
(e)
Active or previous diagnosis of cancer within 6 months—1 point
(f)
Recent surgery ± immobilization within 4 weeks—1.5 points
(g)
Recent history of hemoptysis—1.0 points
Score:
(a)
<2 points—low probability
(b)
2–6 points—moderate probability
(c)
>6 points—high probability
Testing for Pulmonary Embolism
D–Dimer Testing: It is very sensitive but less specific for PE; therefore negative D-Dimer may exclude the presence of PE with great certainty.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


