Electrical cardioversion (ECV) is recommended for rhythm control in patients with atrial arrhythmia; yet, ECV use and outcomes in contemporary practice are unknown. We reviewed all nonemergent ECVs for atrial arrhythmias at a tertiary care center (2010 to 2013), stratifying patients by transesophageal echocardiography (TEE) use before ECV and comparing demographics, history, vitals, and laboratory studies. Outcomes included postprocedural success and complications and repeat cardioversion, rehospitalization, and death within 30 days. Overall, 1,017 patients underwent ECV, 760 (75%) for atrial fibrillation and 240 (24%) for atrial flutter; 633 underwent TEE before ECV and 384 did not. TEE recipients were more likely to be inpatients (74% vs 44%, p <0.001), have higher mean CHADS 2 scores (2.6 vs 2.4, p = 0.03), and lower mean international normalized ratios (1.2 vs 2.1, p <0.001). Overall, 89 patients (8.8%) did not achieve sinus rhythm and 14 experienced procedural complications (1.4%). Within 30 days, 80 patients (7.9%) underwent repeat ECV, 113 (11%) were rehospitalized, and 14 (1.4%) died. Although ECV success was more common in patients who underwent TEE before ECV (77% vs 68%, p = 0.01), there were no differences in 30-day death or rehospitalization rates (11.1% vs 13.0%, p = 0.37). In multivariate analyses, higher pre-ECV heart rate was associated with increased rehospitalization or death (adjusted hazard ratio 1.15/10 beats/min, 95% confidence interval 1.07 to 1.24, p <0.001), whereas TEE use was associated with lower rates (adjusted hazard ratio 0.58, 95% confidence interval 0.39 to 0.86, p = 0.007). In conclusion, failures, complications, and rehospitalization after nonemergent ECV are common and associated more with patient condition than procedural characteristics. TEE use was associated with better clinical outcomes.
In hopes of better understanding electrical cardioversion (ECV) use and outcomes, we sought to (1) describe a contemporary cohort of patients undergoing nonemergent cardioversion at a major tertiary care hospital, with or without preprocedural transesophageal echocardiography (TEE); (2) explore the outcomes in patients undergoing cardioversion; and (3) identify factors associated with adverse outcomes after cardioversion.
Methods
We included consecutive patients who underwent nonemergent ECV at Duke University Medical Center from January 2010 to March 2013. Both inpatient and outpatient procedures were included, as long as they occurred in the cardioversion “suite,” which is a unified location for all nonemergent ECVs at our institution. (Pharmacologic cardioversions are not performed in this setting.) For patients with multiple cardioversions during the study period, the first procedure was included in the analysis as the index event. To identify nonemergent cardioversions unrelated to invasive cardiac procedures or catastrophic protracted hospitalizations, several exclusion criteria were used. Cardioversions performed in the electrophysiology laboratories and those for ventricular arrhythmias were not included. Additionally, to provide more clinically relevant insights, we excluded patients with cardiac surgery or catheter ablation for atrial fibrillation (AF) within 90 days before cardioversion, those with metastatic cancer, and inpatients with hospitalizations for >14 days before cardioversion.
Baseline demographics, medical history, laboratory results, administrative data, and clinical outcomes were derived from the electronic health record and based on clinical diagnosis, including both laboratory and billing systems, through the Decision Support Repository at Duke University. Risk scores for stroke in patients with AF (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke or TIA or thromboembolism [CHADS 2 ] and Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke or TIA or thromboembolism, Vascular disease, Age 65–74 years, Sex category [CHA 2 DS 2 -VASc]) were calculated using previously described methods. Periprocedural data on the cardioversion procedure and immediate outcomes were analyzed from the clinical cardioversion procedure log, which is a database that includes preprocedural vital signs and rhythm, inpatient status, anesthesia details, cardioversion approach, postcardioversion vital signs and rhythm, and immediate complications. The procedure log is part of the medical documentation and record for each patient undergoing ECV at Duke University. Detailed ambulatory and inpatient medication use were not available.
Patient outcomes included immediate periprocedural complications, failed cardioversion, repeat cardioversion, thromboembolic events, rehospitalization (with cause), or death within 30 days. Periprocedural complications were derived from the procedure log and defined as bradycardia requiring treatment (medical or electrical), cardiac arrest, hypotension requiring treatment, significant hypoxia, or additional arrhythmia requiring treatment (e.g., ventricular tachycardia, ventricular fibrillation). Failed cardioversion was defined as any periprocedural complication (as mentioned previously) or a postcardioversion rhythm of AF, atrial flutter, atrial tachycardia, or low atrial rhythm. All repeat hospitalizations and death within the Duke University Health System were captured through the Decision Support Repository, which is an electronic clearinghouse for clinical data within the health system. Cause of hospitalizations and deaths was identified through primary review of the medical record with categorization of events using the primary clinical diagnosis for the hospitalization and cause of death in the death summaries. To identify factors associated with adverse clinical outcomes, we used the composite end point of rehospitalization or death within 30 days of the procedure.
To capture TEEs most likely to be performed in anticipation of cardioversion, we stratified baseline and procedural characteristics by the use of TEE within 7 days before cardioversion. Distribution of TEE timing was assessed to confirm the validity of this approach. Categorical variables are described as number and percentage and compared using Pearson chi-square tests; continuous variables are described as median and twenty-fifth to seventy-fifth percentiles and compared using Wilcoxon rank sum tests.
Immediate postcardioversion characteristics and cardioversion success are described and compared by TEE use, CHADS 2 scores, and CHA 2 DS 2 -VASc scores using similar chi-square tests. Thirty-day rehospitalization or death was evaluated with Kaplan–Meier curves and log-rank tests by these same key variables.
Multivariable Cox proportional hazards regression was used to identify and describe factors associated with death or rehospitalization within 30 days in this patient cohort. Models were derived using backward selection, with a stay criterion of p <0.10. Candidate variables for selection included baseline patient characteristics, medical history, preprocedural vital signs, preprocedural rhythm, use of preprocedural TEE, inpatient status, and cardioversion shock mechanism. All candidate variables were assessed for linearity and proportional hazards assumptions. There were no relevant proportional hazards violations; linearity was addressed using linear splines when needed. Subsequently, we added preprocedural laboratory data as candidate covariates using the same modeling approach; however, this was limited to the subset of 896 patients (88%) with laboratories available within 30 days before the procedure. We further assessed the impact of including immediate postcardioversion results in the model of 30-day outcomes, using the same backward-selection procedure with the added consideration of postcardioversion vitals, postcardioversion rhythm, or immediate postcardioversion complication, as candidate covariates.
This analysis was approved by the Institutional Review Board of Duke University, which granted a waiver of informed consent. All statistical analyses of aggregate, deidentified data were performed at the Duke Clinical Research Institute using SAS software (version 9.2 or greater, SAS Institute, Cary, North Carolina). No extramural funding support was used.
Results
A total of 1,229 patients who underwent nonemergent cardioversion were identified, with a total of 1,663 cardioversion procedures recorded. After applying exclusion criteria, this yielded a final study cohort of 1,017 patients who underwent their first cardioversion during the study period ( Figure 1 ). Overall, 60% (n = 608) of patients were hospitalized as inpatients at the time of their index procedure; the mean time from cardioversion to discharge was 3.5 days.
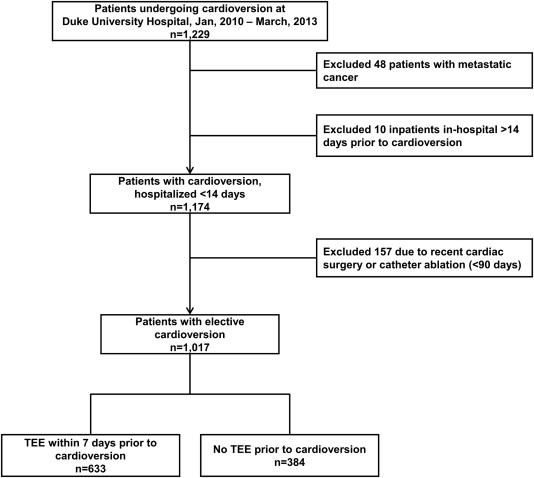
Baseline characteristics and procedural details, stratified by TEE use, are provided in Table 1 . Imaging findings within the cohort are provided in Table 2 , with unadjusted, immediate postcardioversion outcomes in Table 3 . Overall, 8.8% of patients (n = 89) failed to achieve sinus rhythm after cardioversion, and there were 14 immediate complications in 13 patients (1.3%). Rates of ECV failure or complication were not significantly different between patients with versus without TEE (9.5% vs 9.6%, p = 0.93) or by CHADS 2 (9.8% for ≥2 vs 8.7% for 0 to 1, p = 0.59) or CHA 2 DS 2 -VASc scores (9.7% for ≥2 vs 8.2% for 0 to 1, p = 0.61).
| Overall (n=1017) | TEE Prior to Cardioversion (n=633) | Cardioversion without TEE (n=384) | p-value | |
|---|---|---|---|---|
| Age, median (IQR) | 68 (59-76) | 68 (60-76) | 69 (59-76) | 0.7 |
| Female | 351/1016 (35%) | 232/632 (37%) | 119/384 (31%) | 0.06 |
| Inpatient | 632/1017 (62%) | 465/633 (74%) | 167/384 (44%) | <0.001 |
| Hypertension | 858/1017 (84%) | 531/633 (84%) | 327/384 (85%) | 0.6 |
| Diabetes | 342/1017 (34%) | 232/633 (37%) | 110/384 (29%) | 0.009 |
| Renal failure | 265/1017 (26%) | 187/633 (30%) | 78/384 (20%) | 0.001 |
| Smoker | 315/1017 (31%) | 199/633 (31%) | 116/384 (30%) | 0.7 |
| Hyperlipidemia | 659/1017 (65%) | 417/633 (66%) | 242/384 (63%) | 0.4 |
| Heart failure ∗ | 578/1017 (57%) | 381/633 (60%) | 197/384 (51%) | 0.006 |
| Coronary heart disease ∗ | 510/1017 (50%) | 319/633 (50%) | 191/384 (50%) | 0.8 |
| Prior myocardial infarction | 229/1017 (23%) | 145/633 (23%) | 84/384 (22%) | 0.7 |
| Prior cerebrovascular disease | 244/1017 (24%) | 160/633 (25%) | 84/384 (22%) | 0.2 |
| Peripheral vascular disease | 128/1017 (13%) | 86/633 (14%) | 42/384 (11%) | 0.2 |
| Chronic obstructive pulmonary disease | 79/1017 (7.8%) | 57/633 (9.0%) | 22/384 (5.7%) | 0.06 |
| Prior liver disease | 37/1017 (3.6%) | 23/633 (3.6%) | 14/384 (3.6%) | 1.0 |
| CHADS 2 score, mean (SD) | 2.5 (1.5) | 2.6 (1.5) | 2.4 (1.5) | 0.03 |
| CHADS 2 score ≥2 | 742/1017 (73%) | 472/633 (75%) | 270/384 (70%) | 0.1 |
| CHA 2 DS 2 -VASc score, mean (SD) | 4.0 (2.1) | 4.1 (2.1) | 3.9 (2.1) | 0.07 |
| CHA 2 DS 2 -VASc score ≥2 | 906/1016 (89%) | 574/632 (91%) | 332/384 (87%) | 0.03 |
| TEE same day as cardioversion | 575/1017 (57%) | 575/633 (91%) | – | – |
| Pre-cardioversion vital signs | ||||
| Diastolic blood pressure (mm Hg), median (IQR) | 76 (66-86) | 75 (66-86) | 76 (67-86) | 0.6 |
| Systolic blood pressure (mm Hg), median (IQR) | 128 (114-144) | 128 (114-143) | 129 (114-145) | 0.7 |
| Heart rate, median (IQR) | 89 (74-110) | 91 (77-113) | 83 (71-102) | <0.001 |
| Labs prior to cardioversion | ||||
| Hemoglobin (g/dL), median (IQR) | 14 (12-15) | 13 (12-15) | 14 (12-15) | 0.06 |
| Platelets (x10 3 ), median (IQR) | 210 (172-253) | 213 (173-260) | 205 (170-238) | 0.01 |
| International normalized ratio, median (IQR) | 1.4 (1.1-2.3) | 1.2 (1.0-1.9) | 2.1 (1.2-2.7) | <0.001 |
| aPTT (seconds), median (IQR) | 35 (29-42) | 32 (29-39) | 39 (35-44) | <0.001 |
| Creatinine (mg/dL), median (IQR) | 1.1 (0.9-1.4) | 1.1 (0.9-1.4) | 1.1 (0.9-1.4) | 0.04 |
| Potassium (mmol/L), median (IQR) | 4.2 (3.9-4.5) | 4.2 (3.8-4.5) | 4.3 (4.0-4.5) | 0.03 |
| Magnesium (mg/dL), median (IQR) | 2.1 (1.9-2.2) | 2.1 (1.9-2.2) | 2.1 (1.9-2.2) | 0.4 |
| Pre-cardioversion rhythm | 0.007 | |||
| Atrial fibrillation | 760/1017 (75%) | 452/633 (71%) | 308/384 (80%) | |
| Atrial flutter | 240/1017 (24%) | 170/633 (27%) | 70/384 (18%) | |
| Atrial tachycardia | 17/1017 (1.7%) | 11/633 (1.7%) | 6/384 (1.6%) | |
| Cardioversion via implanted device | 12/949 (1.3%) | 6/593 (1.0%) | 6/356 (1.7%) | 0.4 |
| Cardioversion sedation | ||||
| Propofol | 973/1017 (96%) | 608/633 (96%) | 365/384 (95%) | 0.4 |
| Propofol dose (mg), median (IQR) | 110 (80-160) | 140 (100-200) | 80 (60-100) | <0.001 |
| Midazolam | 7/1017 (0.7%) | 4/633 (0.6%) | 3/384 (0.8%) | 1.000 |
| Fentanyl | 11/1017 (1.1%) | 6/633 (0.9%) | 5/384 (1.3%) | 0.8 |
∗ Based on clinical diagnosis codes in the electronic health record, as documented by the treating physician.
| Overall (n=1017) | TEE Prior to Cardioversion (n=633) | Cardioversion without TEE (n=384) | p-value | |
|---|---|---|---|---|
| LA Size | 0.691 | |||
| Normal | 142/691 (20.5%) | 94/448 (21.0%) | 48/243 (19.8%) | |
| Small | 1/691 (0.1%) | 1/448 (0.2%) | 0/243 (0.0%) | |
| Mildly enlarged | 331/691 (47.9%) | 220/448 (49.1%) | 111/243 (45.7%) | |
| Moderately enlarged | 196/691 (28.4%) | 120/448 (26.8%) | 76/243 (31.3%) | |
| Severely enlarged | 21/691 (3.0%) | 13/448 (2.9%) | 8/243 (3.3%) | |
| MV Leaflets | <.001 | |||
| Normal | 784/879 (89.2%) | 563/613 (91.8%) | 221/266 (83.1%) | |
| Abnormal | 95/879 (10.8%) | 50/613 (8.2%) | 45/266 (16.9%) | |
| MV Mobility | 0.525 | |||
| Fully mobile | 846/877 (96.5%) | 591/611 (96.7%) | 255/266 (95.9%) | |
| Partially mobile | 31/877 (3.5%) | 20/611 (3.3%) | 11/266 (4.1%) | |
| Completely immobile | 0/877 (0.0%) | 0/611 (0.0%) | 0/266 (0.0%) | |
| LVEF | 0.750 | |||
| <15% | 33/826 (4.0%) | 23/568 (4.0%) | 10/258 (3.9%) | |
| 20% | 30/826 (3.6%) | 18/568 (3.2%) | 12/258 (4.7%) | |
| 25% | 35/826 (4.2%) | 25/568 (4.4%) | 10/258 (3.9%) | |
| 30% | 25/826 (3.0%) | 18/568 (3.2%) | 7/258 (2.7%) | |
| 35% | 29/826 (3.5%) | 16/568 (2.8%) | 13/258 (5.0%) | |
| 40% | 49/826 (5.9%) | 35/568 (6.2%) | 14/258 (5.4%) | |
| 45% | 53/826 (6.4%) | 36/568 (6.3%) | 17/258 (6.6%) | |
| 50% | 88/826 (10.7%) | 65/568 (11.4%) | 23/258 (8.9%) | |
| >55% | 484/826 (58.6%) | 332/568 (58.5%) | 152/258 (58.9%) | |
| Preserved LVEF (≥50) | 572/826 (69.2%) | 397/568 (69.9%) | 175/258 (67.8%) | 0.551 |
∗ Based on most recent transthoracic or transesophageal echocardiogram within the prior year.
| Variable | Overall (n=1017) | TEE Prior to Cardioversion (n=633) | Cardioversion without TEE (n=384) | p-value |
|---|---|---|---|---|
| Post-cardioversion vital signs | ||||
| Diastolic blood pressure (mm Hg), median (IQR) | 63 (56-72) | 62 (55-70) | 65 (58-74) | <0.001 |
| Systolic blood pressure (mm Hg), median (IQR) | 111 (98-127) | 109 (96-124) | 117 (103-130) | <0.001 |
| Heart rate, median (IQR) | 67 (59-76) | 69 (60-78) | 65 (55-74) | <0.001 |
| Post-cardioversion rhythm | 0.01 | |||
| Atrial fibrillation | 65/1017 (6.4%) | 39 (6.2%) | 26 (6.8%) | |
| Atrial flutter | 11/1017 (1.1%) | 6 (0.9%) | 5 (1.3%) | |
| Atrial tachycardia | 1/1017 (0.1%) | 1 (0.2%) | 0 (0.0%) | |
| Low atrial rhythm | 12/1017 (1.2%) | 7 (1.1%) | 5 (1.3%) | |
| Normal sinus rhythm | 748/1017 (74%) | 488 (77%) | 260 (68%) | |
| Sinus bradycardia | 180/1017 (18%) | 92 (15%) | 88 (23%) | |
| Any complication ∗ | 13/1017 (1.3%) | 11/633 (1.7%) | 2/384 (0.5%) | 0.1 |
| Bradycardia requiring treatment | 4 (0.4%) | 4 (0.6%) | 0 | |
| Cardiac arrest | 1 (0.1%) | 1 (0.2%) | 0 | |
| Hypotension requiring treatment | 4 (0.4%) | 3 (0.5%) | 1 (0.3%) | |
| Hypoxia | 2 (0.2%) | 2 (0.3%) | 0 | |
| VT/VF requiring treatment | 1 (0.1%) | 0 | 1 (0.3%) | |
| Other arrhythmia | 2 (0.2%) | 2 (0.3%) | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


