The objective of the present study was to characterize the outcomes and resource utilization of all infants born with hypoplastic left heart syndrome (HLHS) in the Intermountain West. This was a retrospective cohort study of all infants born with HLHS in the Intermountain West from January 1995 and January 2010. The cohort was divided into 3 eras: era 1, 1995 to 1999; era 2, 2000 to 2004; and era 3, 2005 to 2010. Cox proportional hazards regression analysis was performed to assess mortality. The lifetime hospitalization days and charges were also determined. Of the 245 infants identified, 65% were male infants and 172 (70%) underwent Stage 1 palliation. The transplant-free survival rate for the entire cohort was 33% at 14 years. The 1-year transplant-free survival rate for the surgical cohort was 60% in era 3. The infants whose initial presentation included shock, restrictive or intact atrial septum, chromosomal defects, or multiorgan dysfunction had an increased risk of death. A recent era of birth, greater birthweight, and older gestational age were associated with improved survival. The factors associated with mortality after stage 1 included surgical procedure type (Blalock-Taussig vs Sano shunt, hazard ratio 2.1), requirement for postoperative extracorporeal membrane oxygenation (hazard ratio 4.2), postoperative renal dysfunction (hazard ratio 3.0), anomalous pulmonary venous return (hazard ratio 2.9), and moderate or greater tricuspid valve regurgitation at any point (hazard ratio 2.0). For patients who had undergone stage 1, 2, or 3 palliation, the median cumulative lifetime hospitalization was 32, 48, and 65 days, and the median cumulative lifetime charges for hospitalization were $201,812, $253,183, and $296,213, respectively. In conclusion, although hospital-based studies of HLHS have shown significantly improved survival after surgical palliation, population-based studies have shown that HLHS continues to have a high mortality and high resource utilization.
The state of Utah and parts of the adjoining states in the Intermountain West region are served by 1 congenital heart surgery program, and the follow-up rates for patients with hypoplastic left heart syndrome (HLHS) have been >95%. Unlike data from administrative databases, this provides a unique opportunity to study the contemporary outcomes, surgical complications, and resource utilization of all patients born with HLHS in the Intermountain West. The present study reports on the outcomes and resource utilization of all infants born with HLHS in the state of Utah and the surrounding Intermountain West from January 1995 to January 2010.
Methods
The present study was a retrospective review of all infants born with HLHS from January 1995 to January 2010 in Utah and the Intermountain West region. The institutional review boards at the University of Utah, Primary Children Medical Center, and the Utah Department of Health approved the study.
The study cohort was identified from the Utah Birth Defect Network (UBDN), Enterprise Data Warehouse (EDW) of the Intermountain Health Care, University of Utah Fetal Heart Center database, and University of Utah Pediatric Cardiothoracic Surgery database. The UBDN is an active surveillance statewide birth defects registry that collects information on children from 20 weeks of gestation to 2 years of age. All Utah hospitals and birthing centers are required to report a specific set of information to the UBDN when an infant is born with a birth defect. Each reported case is then actively confirmed and collected by a UBDN staff member. The UBDN started collecting data on HLHS in 1999, and the outcomes records were available through 2009. Liveborn infants with HLHS before 1999 and after 2009 were obtained from the other databases mentioned. The EDW is the electronic administrative data set of Intermountain Health Care and contains data for every inpatient and outpatient encounter, including hospitalizations, procedures, and charges from the 23 hospitals in the Intermountain Healthcare System, including the single children’s hospital with a congenital heart surgery program.
The data elements abstracted from the EDW, UBDN, and the patients’ clinical records included (1) prenatal diagnosis; (2) pregnancy outcomes; (3) birth year; (4) birth history; (5) presentation in shock (hypotension) with metabolic acidosis (pH ≤7.2) that required intervention; (6) delayed diagnosis (>24 hours after birth); (7) multiorgan dysfunction at presentation; (8) preoperative necrotizing enterocolitis; (9) preoperative septicemia; (10) cardiac anatomy; including restrictive or intact atrial septum (for statistical analysis, the study cohort was divided into mitral and aortic atresia [cohort with no discernible left ventricular cavity] and mitral stenosis with aortic atresia/stenosis [cohort with hypoplastic left ventricular cavity]); (11) chromosomal defects; (12) associated anomalies and extracardiac defects; (13) surgical palliation type; (14) postoperative death (within 30 days of surgery); (15) surgical and other complications, including but not limited to, the postoperative need for chest re-exploration, revision of shunt, renal dysfunction (defined as an increase in serum creatinine >1 mg/dl greater than baseline and/or requiring dialysis), need for extracorporeal membrane oxygenation (ECMO), cardiac arrest, rhythm disturbances, need for pacemaker, cardiac catheterizations and interventions, sternal wound infections, septicemia, chylothorax, and so forth; (16) cause of death; (17) cardiac transplantation; (18) right ventricular dysfunction (greater than mild, as subjectively assessed by the reading physician on routine 2-dimensional echocardiogram) at any time; (19) tricuspid valve regurgitation (greater than mild, as subjectively assessed by the reading physician on routine 2-dimensional echocardiogram) at any time; (20) tricuspid valve repair; (21) lifetime length of hospital stay; (22) lifetime length of intensive care unit stay; (23) total lifetime charge of inpatient care (charge was derived from EDW); and (24) interval to death or cardiac transplantation. Outliers (charges exceeding 2 SDs greater than the mean hospital charge for the cohort) were excluded from cost analysis. The charge of hospitalization was corrected for inflation using Tom’s inflation calculator (available at: http://www.halfhill.com/inflation.html ) before statistical analysis.
The incidence of HLHS was calculated using the Utah-born infants and the number of births per year from the Utah census. The full study cohort was divided in to 3 eras according to the child’s year of birth: era 1, 1995 to 1999; era 2, 2000 to 2004; and era 3, 2005 to 2010. Kaplan-Meier survival curves were generated to determine the interval to death or cardiac transplantation. For the survival analysis, cardiac transplantation was considered cardiac death. The 95% confidence intervals (CIs) for the probability of survival were computed. Univariate Cox proportional hazards regression analysis was used to calculate the transplant-free survival for 2 groups: all infants born with HLHS and all infants who underwent palliation for HLHLS. Next, the unadjusted effects of the covariates on mortality were calculated. Only variables that were statistically significant (p <0.05) in the unadjusted (crude) analyses were included in the multivariate analysis. Multivariate Cox proportional hazards modeling was used to assess the adjusted effect of each factor on survival. Multivariate logistic regression analysis was used to estimate the adjusted odds ratio of death with 95% CIs after controlling for confounding variables.
Results
A total of 245 infants born with HLHS during the study period were the study cohort. Of these, 199 (81.2%) were born in Utah and 46 were born in 1 of 5 additional states (Wyoming, Montana, Idaho, Nevada, and Colorado). In Utah, the UBDN identified 168 liveborn patients with HLHS from 1999 to 2009, and the remaining 31 patients were identified from other databases as discussed in the “Methods” section. The birth outcomes of all infants with HLHS in Utah from 1999 to 2009 are listed in Table 1 . The birth history of all liveborn HLHS infants during the study period (n = 245) is listed in Table 2 , with 48 born in era 1, 74 in era 2, and 123 in era 3. The rates of prenatal diagnosis improved from 32% in era 1 to 62% in era 2 and 72% in era 3 (p = 0.02, for era 1 vs era 3). Patient presentation in shock or with acidosis decreased from 27% in era 1 to 14% in era 2 and 9% in era 3 (p = 0.01, for era 1 vs era 3). The incidence of delayed diagnosis (>24 hours after birth) was 27% in era 1, 15% in era 2, and 19% in era 3 (p = NS). The anatomic diagnoses of the study cohort are listed in Table 2 . “Classic” HLHS was seen in 83% of the infants.
| Birth Year | Live Births | Stillborn | Termination | Spontaneous Abortion | Total | Live Births/y | Rate/10,000 Live Births | 95% CI |
|---|---|---|---|---|---|---|---|---|
| 1999 | 12 | 2 | 4 | 0 | 18 | 46,243 | 2.60 | 1.34–4.53 |
| 2000 | 13 | 2 | 2 | 1 | 18 | 47,331 | 2.75 | 1.46–4.70 |
| 2001 | 11 | 1 | 6 | 2 | 20 | 47,915 | 2.30 | 1.15–4.11 |
| 2002 | 17 | 1 | 2 | 1 | 21 | 49,140 | 4.27 | 2.65–6.53 |
| 2003 | 11 | 3 | 1 | 0 | 15 | 49,834 | 3.01 | 1.69–4.96 |
| 2004 | 16 | 2 | 0 | 0 | 18 | 50,653 | 3.55 | 2.11–5.62 |
| 2005 | 19 | 1 | 0 | 0 | 20 | 51,517 | 3.88 | 2.37–6.00 |
| 2006 | 14 | 1 | 0 | 0 | 15 | 53,475 | 2.81 | 1.57–4.63 |
| 2007 | 18 | 1 | 2 | 0 | 21 | 55,063 | 3.81 | 2.36–5.83 |
| 2008 | 19 | 1 | 0 | 0 | 20 | 55,605 | 3.60 | 2.20–5.55 |
| 2009 | 18 | 1 | 1 | 0 | 20 | 53,849 | 3.71 | 2.27–5.74 |
| Total | 168 (81) | 16 (8) | 18 (9) | 4 (2) | 206 | 560,625 | 3.3 ± 0.6 |
| Variable | Value |
|---|---|
| Mean birthweight (kg) | 3.1 (1.1–4.7) |
| Mean gestational age (weeks) | 38 (27–42) |
| Males (n) | 160 (65%) |
| Singelton | 237 (97%) |
| Twins | 7 (3%) |
| Triplets | 1 (<1%) |
| Mitral atresia and aortic atresia | 79 (32%) |
| Mitral stenosis and aortic stenosis | 70 (29%) |
| Mitral stenosis and aortic atresia | 55 (22%) |
| Mitral atresia and aortic stenosis (hypoplastic left heart syndrome with ventricular septal defect and double outlet right ventricle) | 22 (9%) |
| Unbalanced atrioventricular canal defect | 13 (5%) |
| Heterotaxy syndrome with unbalanced atrioventricular canal defect | 7 (3%) |
| Other hypoplastic left heart syndrome variants | 6 (2%) |
| Atrial septal restriction | 19 (8%) |
| Intact atrial septum | 5 (2%) |
| Anomalous pulmonary venous connection | 17 (7%) |
| Pulmonary venous anomalies without heterotaxy syndrome | 10 (4%) |
| Pulmonary venous anomaly with heterotaxy syndrome | 7 (3%) |
| Pulmonary vein stenosis | 8 (3%) |
| Left superior vena cava | 14 (6%) |
| Levoatriocardinal vein | 2 (1%) |
| Coronary sinusoids ⁎ | 14 (6%) |
⁎ Communication between coronary arteries and cardiac chamber (coronary–cameral fistulas).
Chromosomal anomalies were rare (2%, n = 6) and included Turner syndrome in 3, trisomy 21 in 1, trisomy 18 in 1, trisomy 7 with 11q deletion in 1, and CHARGE syndrome in 2. Extracardiac anomalies in the absence of a chromosomal defect or definable syndrome were seen in 29 infants (12%). Central nervous system malformations were the most common extracardiac defects (n = 12; 5%) and included agenesis of the corpus callosum, abnormality of the cortical mantel, holoprosencephaly, and micrencephaly. Other extracardiac defects included genitourinary defects (n = 10; 4%), gastrointestinal defects (n = 4; 2%), ophthalmologic defects (n = 3; 1%), and miscellaneous musculoskeletal defects (n = 4; 2%). The studies for chromosomal defects or comprehensive screening for extracardiac defects were not routinely performed in all patients.
Of the 245 infants born with HLHS during the study period, 172 (70%) underwent surgical palliation ( Table 3 ). In our center, since 2005, the Norwood procedure with a Sano shunt has replaced the Norwood procedure with the modified Blalock-Taussig (MBT) shunt as the preferred modality of stage 1 surgical palliation. In our center, the hybrid procedure (bilateral pulmonary artery bands, a ductal stent, and atrial septostomy) is primarily reserved for high-risk infants with co-morbidities as a bridge to transplantation or delaying cardiopulmonary bypass. The type of stage 1 palliation, interval to palliation, and the surgical and interstage mortality stratified by era are listed in Table 3 . An extracardiac fenestrated conduit was placed in all 47 patients who underwent the modified Fontan procedure.
| Variable | Era | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Stage 1 palliation | 17 (10%) | 55 (32%) | 100 (58%) | 172 (100%) |
| Norwood with Sano shunt | 0 | 12 (7%) | 81 (47%) | 93 (54%) |
| Norwood with modified Blalock-Taussig shunt | 17 (10%) | 42 (24%) | 15 (9%) | 74 (43%) |
| Hybrid procedure | 0 | 1 (<1%) | 4 (2%) | 5 (3%) |
| Interval to stage 1 (days) | 13.9 ± 7.5 | 7.8 ± 4.9 | 6.8 ± 3.6 | |
| Stage 1 mortality within 30 days | 7 (41%) | 18 (33%) | 14 (14%) | 39 (23%) |
| Mortality between stage 1 and 2 | 1 (10%) | 5 (14%) | 12 (14%) | 18 (14%) |
| Stage 2 palliation | 9 (8%) | 34 (32%) | 65 (60%) | 107 (62%) |
| Age at stage 2 palliation (months) | 6.4 ± 1.7 | 5.5 ± 1.5 | 5.4 ± 2.1 | |
| Stage 2 mortality (within 30 days) | 1 (1%) | 0 | 0 | |
| Mortality between stage 2 to 3 palliation | 1 (13%) | 2 (6%) | 10 (13%) | 13 (12%) |
| Stage 3 palliation | 5 (11%) | 22 (47%) | 20 (42%) | 47 (27%) |
| Age at stage 3 palliation (months) | 37 ± 10 | 35 ± 10 | 33 ± 10 | |
| Stage 3 mortality within 30 days | 0 | 1 (2%) | 0 | |
Of the 172 patients who underwent surgical palliation, complete follow-up data were available for 167 (97%). The median duration of follow-up was 48.4 months (range 3 to 170). In the absence of any co-morbidities, the parents of 19 infants (8%) chose comfort care before stage 1. For the remaining 54 infants (22%), the parents chose comfort care secondary to co-morbidities (e.g., severe prematurity, multiorgan dysfunction).
The causes of death are listed in Table 4 . Of the 245 total infants born with HLHS, 148 (61.6%) died or required cardiac transplantation. Of the 24 patients listed, 15 (62.5%) underwent cardiac transplantation, 7 (29%) died waiting, and 2 (8.3%) remained on the transplant waiting list.
| Variable | n (%) |
|---|---|
| Total deaths | 148 |
| Postoperative deaths (within 30 days after surgery) | 41 (17%) |
| Palliative care | 43 (29%) |
| Palliative care owing to co-morbidities before or after stage 1 | 24 (16%) |
| Palliative care without co-morbidities before stage 1 | 19 (13%) |
| Cardiac transplantation | 15 (10%) |
| Delayed diagnosis and multiorgan dysfunction | 11 (7%) |
| Sudden cardiac death | 10 (7%) |
| Preoperative arrest | 4 (3%) |
| Pulmonary vein stenosis | 4 (3%) |
| Postcardiac catheterization | 3 (2%) |
| Anomalous pulmonary venous return | 3 (2%) |
| Atrial septal restriction | 3 (2%) |
| Pulmonary arterial hypertension | 2 (1%) |
| Sano thrombus | 1 (<1%) |
| Viral pneumonia | 1 (<1%) |
| Other (renal failure, birth asphyxia, gastrointestinal bleeding) | 7 (5%) |
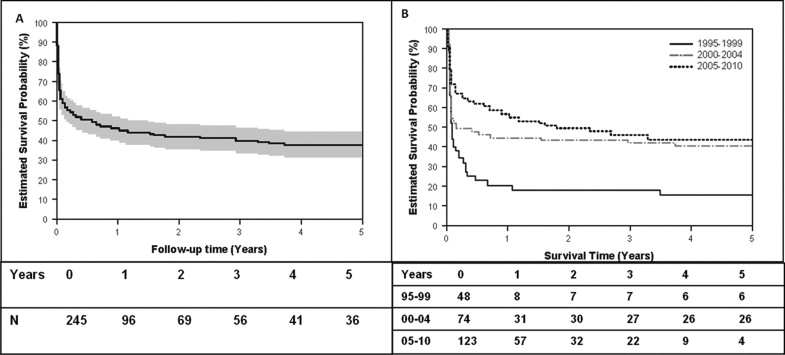
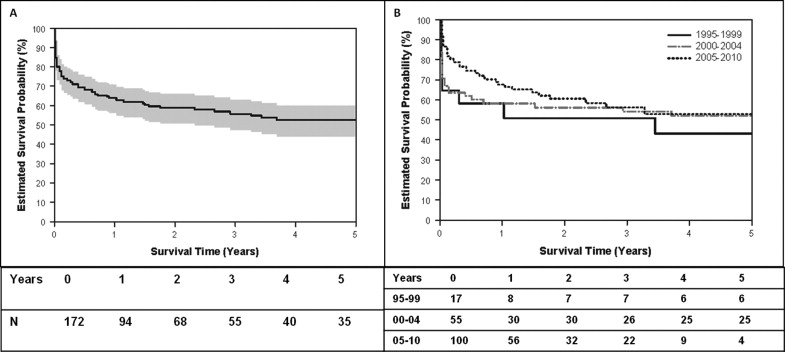
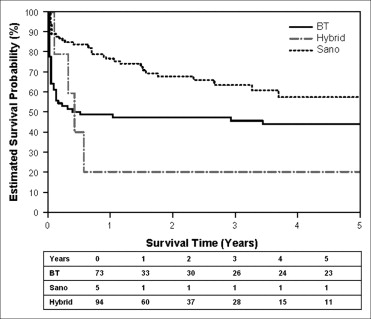
Of the 92 infants who survived, the median survival was 165 days and the estimated transplant-free survival probability rate was 33% at year 14. The transplant-free survival of all infants born with HLHS and the transplant-free survival of all infants born with HLHS stratified by era of birth are shown in Figure 1 . Transplant-free survival for the overall cohort improved from 17% in era 1 to 41% in era 2 and 49% in era 3. The overall transplant-free survival of the cohort who underwent stage 1 palliation and survival stratified by era are shown in Figure 2 . The 1-year transplant-free survival for the entire surgical cohort was 56% (n = 96) and improved from 47% in era 1 to 51% in era 2 and 60% in era 3. The survival of patients stratified by the type of stage 1 palliation is shown in Figure 3 . The overall transplant-free survival in the Sano shunt group was significantly better (68%) than that in the MBT group (45%; hazard ratio 2.1, 95% CI 1.3 to 3.5, p = 0.0023). Of the 5 patients undergoing a hybrid procedure, 1 survived to Fontan, 3 underwent cardiac transplantation, and 1 died waiting for transplantation.
The results of multivariate analysis using Cox proportional hazard modeling with adjusted variables that affected mortality for entire cohort (n = 245) and HLHS infants undergoing surgical palliation (n = 172) are listed in Table 5 . In the entire cohort, an earlier era of birth, lower birthweight, younger gestational age, chromosomal defects, intact or restrictive atrial septum, and multiorgan dysfunction at any point were associated with an increased risk of death. In the surgical cohort, the use of a modified Blalock-Taussig shunt, anomalous pulmonary venous return, need for ECMO, postoperative renal dysfunction, tricuspid valve repair, moderate or greater tricuspid regurgitation at any point, and presentation in shock or with acidosis were associated with a greater risk of death. Other factors, including anatomic subtype of HLHS and pre- and postoperative complications such as arrhythmias, catheter interventions, wound infection, recurrent laryngeal nerve dysfunction, or thrombosis, did not affect survival in the present study cohort.
| Variable | p Value | HR | 95% CI |
|---|---|---|---|
| All patients (n = 245) | |||
| Era 3 versus era 1 | 0.0032 | 0.527 | 0.344–0.807 |
| Birthweight (kg; per 1-kg increase) | 0.0175 | 0.674 | 0.487–0.933 |
| Gestational age at birth (weeks; per 1-week increase) | 0.012 | 0.832 | 0.56–1.01 |
| Intact atrial septum | 0.0005 | 5.057 | 2.033–12.580 |
| Multiorgan dysfunction at any point | 0.0091 | 2.439 | 1.248–4.767 |
| Restrictive atrial septum | 0.0400 | 1.806 | 1.027–3.176 |
| Chromosomal defects | 0.0030 | 1.702 | 1.016–3.246 |
| Stage 1 palliation (n = 172) | |||
| Surgery type | 0.0093 | ||
| Modified Blalock-Taussig compared to Sano shunt | 0.0023 | 2.142 | 1.314–3.492 |
| Hybrid procedure compared to Sano shunt | 0.3569 | 1.647 | 0.570–4.762 |
| Extracorporeal membrane oxygenation (yes vs no) | 0.001 | 4.23 | 2.11–8.46 |
| Postoperative renal dysfunction (yes vs no) | 0.0023 | 3.051 | 1.488–6.254 |
| Anomalous pulmonary venous return (yes vs no) | 0.0039 | 2.942 | 1.414–6.123 |
| Tricuspid valve repair (yes vs no) | 0.0175 | 0.230 | 0.069–0.773 |
| Moderate or greater tricuspid regurgitation (at any point; (yes vs no) | 0.0055 | 2.000 | 1.226–3.265 |
| Presentation in shock or with acidosis | 0.0239 | 1.697 | 1.073–2.686 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


